Switching the Cyclization Mode: Chemoenzymatic Synthesis of Lariat Lipopeptides
Published in Chemistry

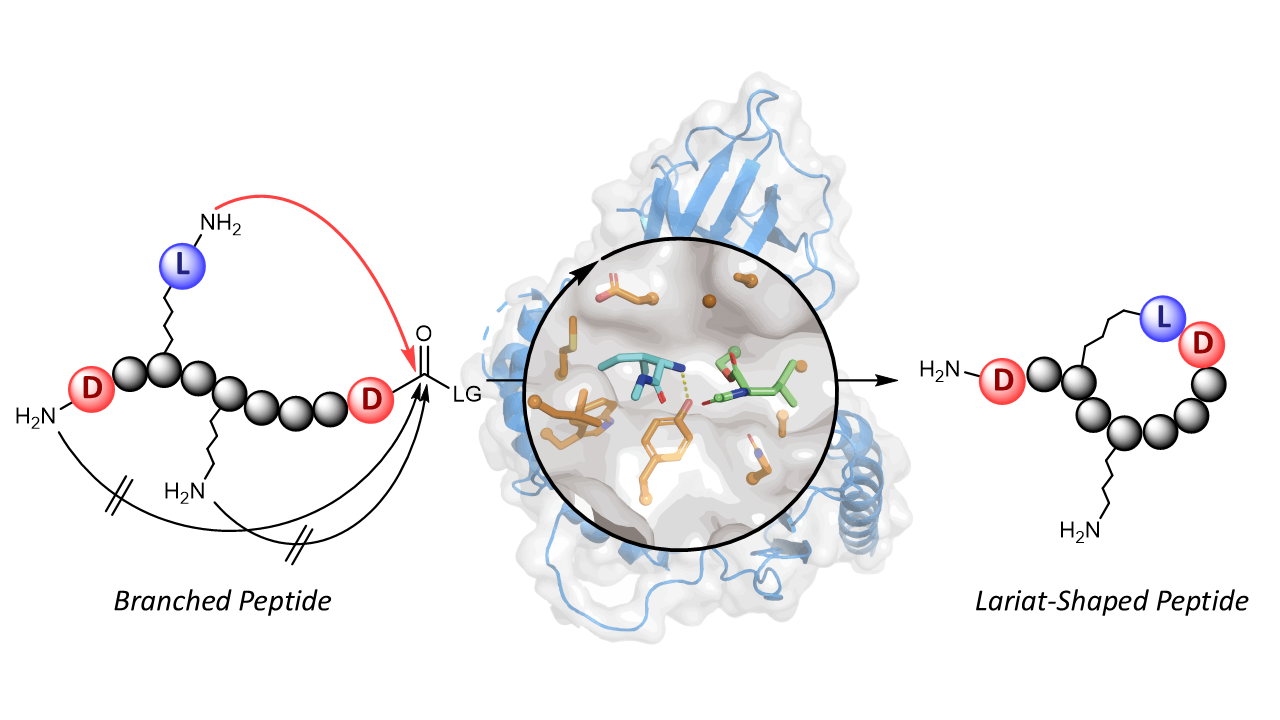
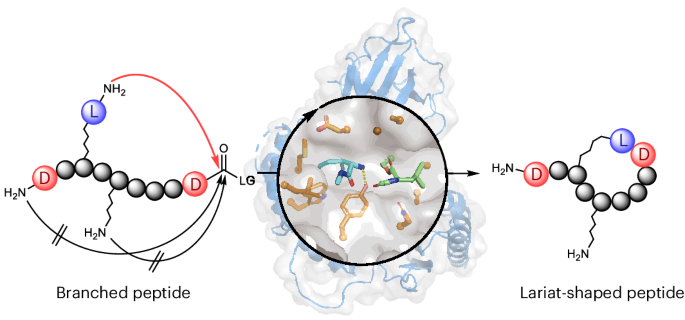
Lariat-shaped lipopeptides—macrocyclic peptides bearing a long fatty acid tail—represent a fascinating class of antibiotics, exemplified by daptomycin and colistin. They act on bacterial cell-surface targets, but their complex architectures make chemical synthesis extremely challenging. We wondered whether nature’s own biosynthetic machinery could be harnessed to construct these intricate molecules in a simpler and modular way.
Turning Cyclases Sideways
Non-ribosomal peptide synthetases (NRPSs) are large multi-enzyme assemblies in nature that produce structurally diverse peptides, including lariat-shaped lipopeptides.[1] Thioesterase (TE) domains catalyze the final cyclization step, closing the peptide chain to form a macrocyclic scaffold. Many researchers have explored TEs as biocatalysts for peptide cyclization; however, lariat-forming TEs typically show narrow substrate specificity and cannot be readily applicable to generate diverse lariat peptides. [2]
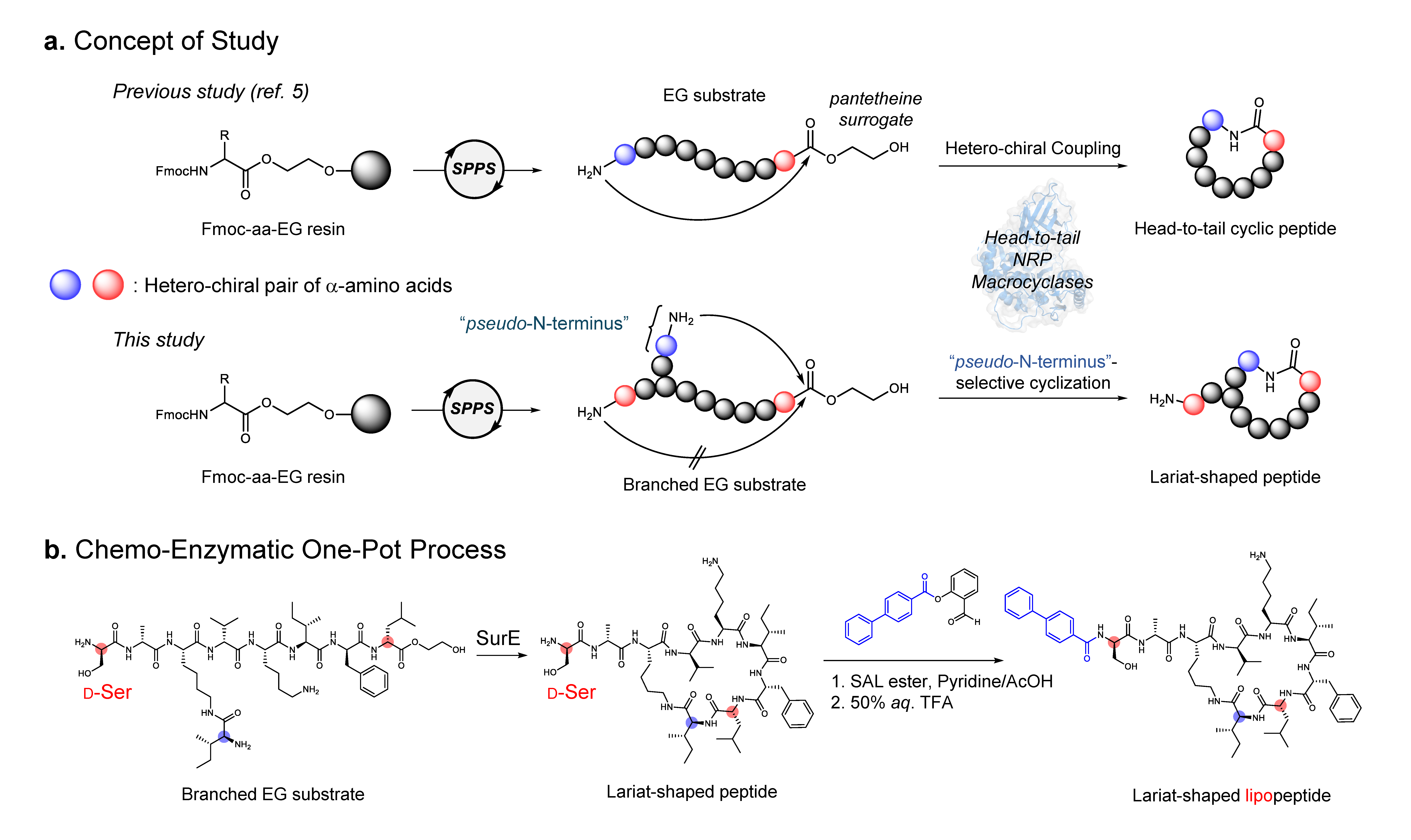
Our team previously discovered penicillin-binding protein-type thioesterases (PBP-type TEs), a newly identified enzyme family of non-ribosomal peptide cyclases. [3,4] These PBP-type TEs are prolific biocatalysts that can generate a wide variety of cyclic peptides in vitro (Figure a). [5] However, they exclusively catalyze head-to-tail macrocyclization by joining both peptide termini, meaning that a versatile biocatalytic approach for lariat peptide synthesis has remained beyond the reach of known NRPS cyclases.
In this study reported in Nature Chemistry entitled as “Non-Ribosomal Peptide Cyclases-Directed Chemoenzymatic Synthesis of Lariat Lipopeptides” (https://www.nature.com/articles/s41557-025-01979-6), we aimed to reprogram a versatile head-to-tail cyclase for a new task: lariat peptide synthesis. Instead of extensively engineering the enzyme itself, we focused on redesigning its substrate. By introducing a “pseudo-N-terminus”, a dipeptide unit featuring an additional N-terminus within the peptide side chain, we created two possible sites for cyclization. Remarkably, the enzyme SurE generated not only the conventional head-to-tail macrocycle but also a lariat-shaped peptide. This straightforward substrate redesign demonstrated that a head-to-tail cyclase can be effectively repurposed to construct lariat peptides (Figure b).
A Stereochemical Switch
To make lariat peptide formation exclusive, we turned to stereochemical control. Because PBP-type TEs accept only l-configured nucleophiles, replacing the peptide’s original N-terminal residue with its mirror-image d-amino acid effectively blocked the typical head-to-tail pathway. This forced the enzyme to use the pseudo-N-terminus as the nucleophile, yielding lariat peptides with complete selectivity.
Interestingly, this strategy was not limited to a single enzyme. Other macrocyclases, such as WolJ and TycC-TE, displayed similar adaptability. Each could be repurposed to form lariat macrocycles through substrate design alone, without any protein engineering.
A One-Pot Route to Lipopeptides and Downstream Evaluation
Because enzymatic transformations proceed with high selectivity, the resulting reaction mixtures are sufficiently pure to bypass product isolation before subsequent modification or biological evaluation (e.g., antimicrobial testing), significantly streamlining the workflow.
Lariat lipopeptides are characterized not only by their macrocyclic head but also by their lipophilic tails, which are essential for biological activity. To integrate these features, we combined enzymatic macrocyclization with serine/threonine ligation (STL), a site-selective acylation reaction targeting the free N‑terminus not used in the cyclization. Remarkably, both reactions—cyclization and acylation—proceeded sequentially in a single pot under mild conditions, eliminating intermediate purification and enabling efficient parallel synthesis of lariat lipopeptides. This streamlined process generated a 51-member library of lariat lipopeptides that could be directly screened for antimicrobial activity against Mycobacterium intracellulare, Mycobacterium abscessus, Staphylococcus aureus, and Escherichia coli. Eight of these compounds inhibited M. intracellulare growth by 50% at concentrations of 8–16 μg ml-1.
Overall, this study highlights the remarkable catalytic promiscuity of certain non‑ribosomal peptide cyclases and emphasizes the advantages of leveraging such biosynthetic enzymes to access natural product‑like compound libraries, ultimately enabling rapid hit discovery. Nature has evolved extraordinarily sophisticated ways of molecular transformation (as exemplified by peptide cyclases but not limited to them) and these mechanisms undoubtedly deserve deeper exploration as tools for synthetic purposes.
References
- Süssmuth, R. D., Mainz, A. Nonribosomal peptide synthesis principles and prospects. Angew. Chem., Int. Ed. Engl. 56, 3770–821 (2017).
- Matsuda, K. Macrocyclizing-thioesterases in bacterial non-ribosomal peptide biosynthesis. J. Nat. Med. 79, 1–14 (2025).
- Kuranaga, T., Matsuda, K., Sano, A., et al. Total synthesis of the nonribosomal peptide surugamide B and identification of a new offloading cyclase family. Angew. Chem., Int. Ed. Engl. 57, 9447–9451 (2018).
- Matsuda, K., Zhai, R., Mori, T., et al. Heterochiral coupling in non-ribosomal peptide macrolactamization. Nat. Catal. 3, 507–515 (2020).
- Kobayashi, M., Fujita, K., Matsuda, K., Wakimoto, T. Streamlined chemoenzymatic synthesis of cyclic peptides by non-ribosomal peptide cyclases. J. Am. Chem. Soc. 145, 3270–3275 (2023).
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in