Many bacteria use cell‐to‐cell signalling, often called quorum sensing (QS), to modulate their activities in response to population density or confinement to niches. QS is a process akin to a bacterial ‘conversation’ by which diffusible signal molecules released into the environment are perceived by different sensors which activate signal transduction pathways that lead to alteration in bacterial gene expression and behaviour. Such cell‐to‐cell signalling is activated above a certain threshold level of the signal molecules and acts to regulate diverse processes that include the formation of biofilms and the production of virulence factors in pathogenic bacteria. The signal molecules produced by bacteria belong to a range of chemical classes. Over the last decade it has become evident that in addition to intraspecies signalling, bacteria use the same signal molecules for interspecies signalling, which may occur in polymicrobial environments to include infections. As with intraspecies signalling, the perception of signals requires a specific receptor and activates processes that may contribute to bacterial persistence, increased tolerance to antibiotics and/or virulence. As a consequence, interference with signalling may afford routes to disease control, by reducing virulence and through improvement of efficacy of existing antibiotic therapies.
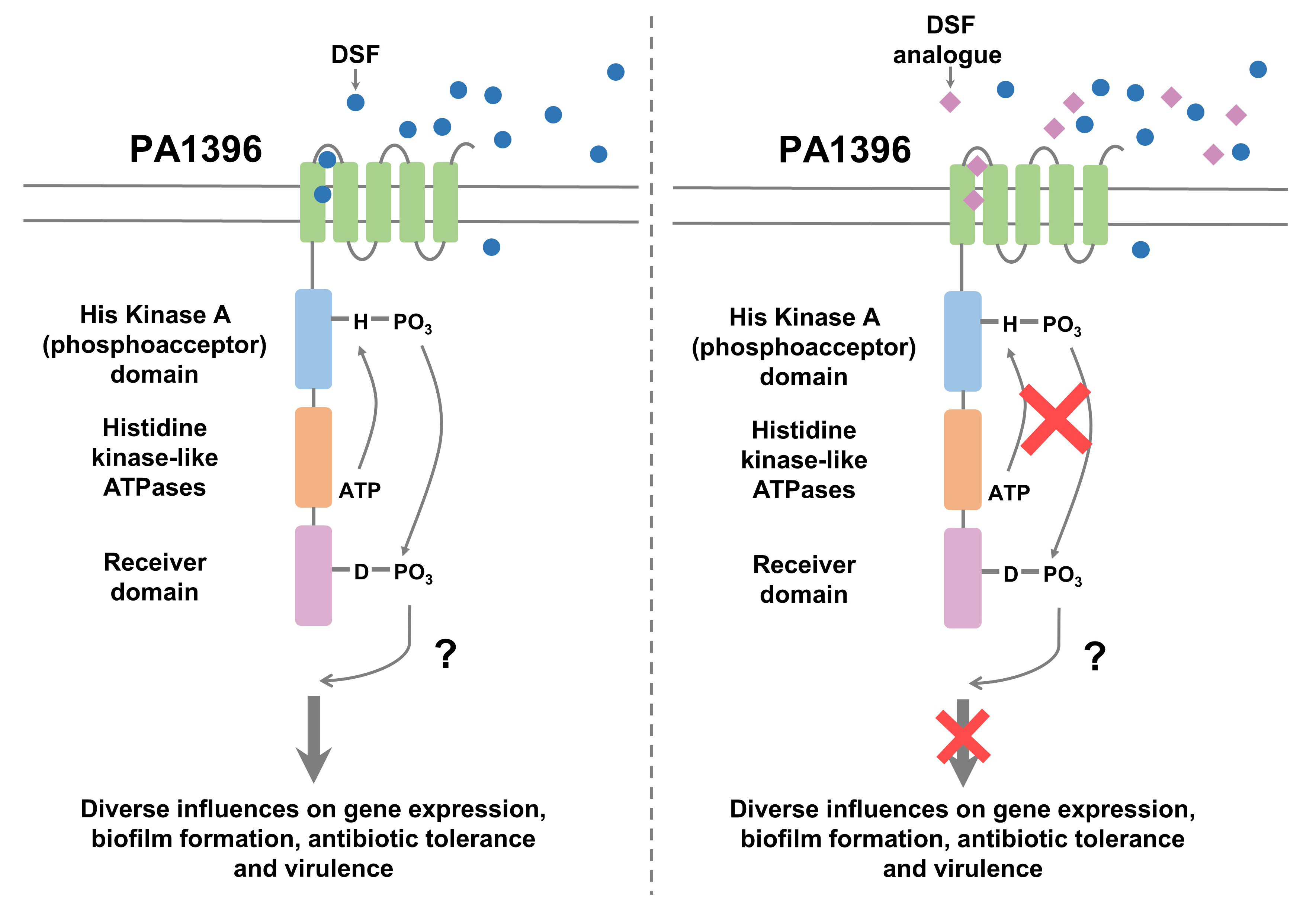
In previous work we showed that the opportunistic pathogen Pseudomonas aeruginosa can participate in interspecies signalling with other bacteria mediated by molecules of the DSF (diffusible signal factor) family, which are cis-2-unsaturated fatty acids. Sensing of these signals involves the histidine kinase PA1396 and leads to altered biofilm formation and increased tolerance to a number of antibiotics. In the current work we examined the ability of a panel of DSF structural analogues to modulate or inhibit PA1396 action as a potential approach to improve the efficacy of existing antibiotics. We established that that the membrane-associated sensory input domain of PA1396 has five trans-membrane helices (TMH). By using a DSF-activated reporter in a series of strains with progressive deletions of this region, we could show that TMH IV and V are required for DSF sensing. In a liposome assay, DSF binding was associated with enhanced auto-phosphorylation of PA1396. Some of the analogue molecules tested blocked DSF action in triggering both specific gene expression and auto-phosphorylation. Other analogues acted as inverse agonists however, to reduce biofilm formation and antibiotic tolerance in laboratory and clinical strains of P. aeruginosa, even in the absence of DSF. Furthermore, we were able to show that one of this latter group of compounds reduced the tolerance of P. aeruginosa to tobramycin in a mouse infection model. Overall the findings suggesting that some analogues may represent promising lead compounds for novel antibiotic adjuvants.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in