InterTissueDC: Exploring gene-gene coupling across brain regions in Alzheimer’s disease
Published in Neuroscience, Protocols & Methods, and Genetics & Genomics
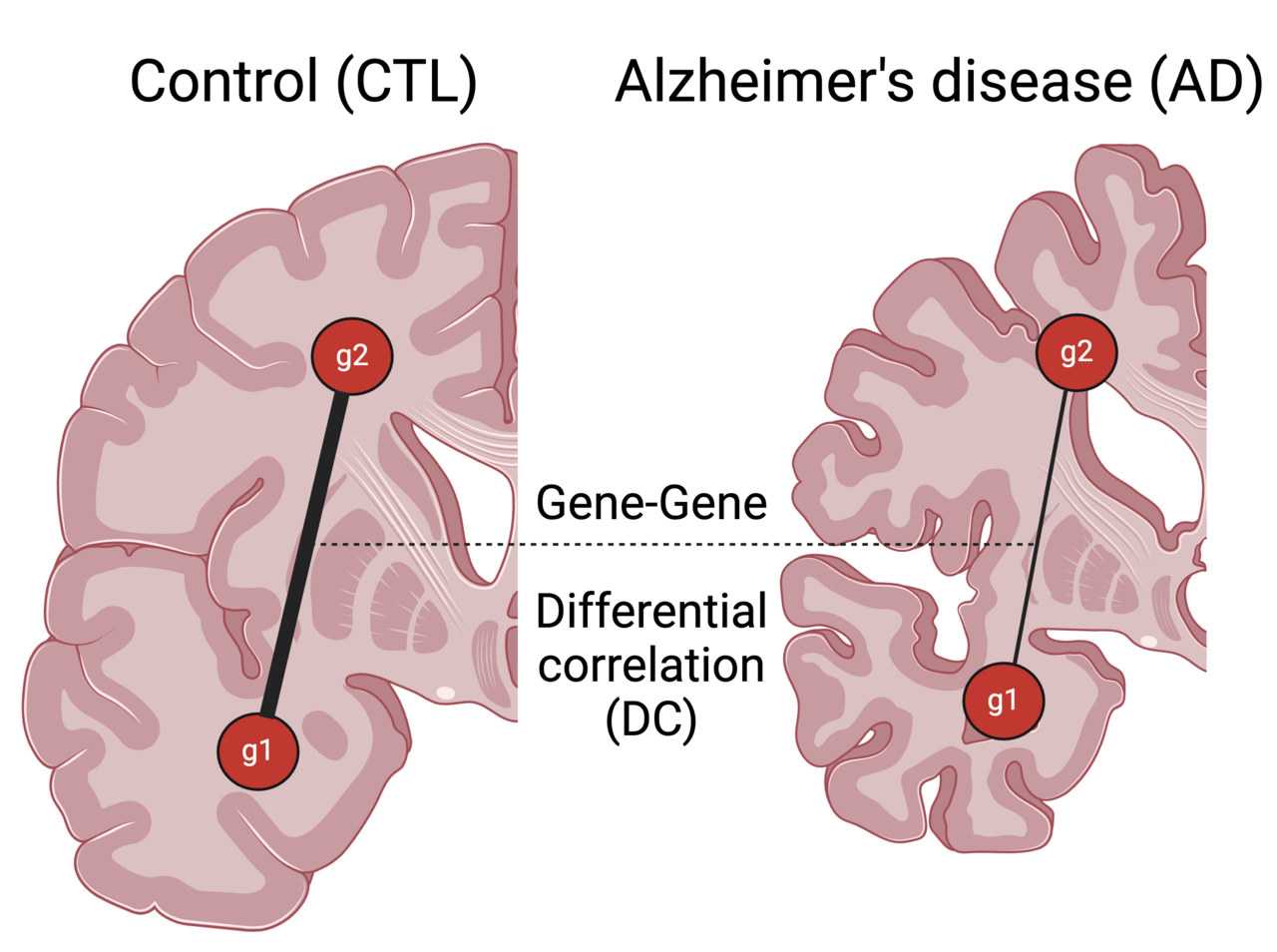
Why study Alzheimer’s disease?
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that presents a significant challenge to our ageing populations. It not only leads to a high mortality rate but also imposes a substantial economic burden on patients, caregivers and the healthcare system. Despite recent advances in clinically approved drugs that target misfolded amyloid plaques, there remains no cure or therapeutic to halt its progression. Our study introduces an innovative approach to better understand and potentially address this complex disease.
Why study networks?
Network analysis in transcriptomics data, where each node in the network is a gene, and the edge weight is usually the correlation between those two genes, has helped better understand the large-scale dysregulations induced by the disease. Downstream network analysis, such as hub analysis, which finds genes that are connected to many other genes, has helped nominate putative therapeutic candidates and led to a better understanding of the disease's biological processes.
What is the innovative idea behind this work?
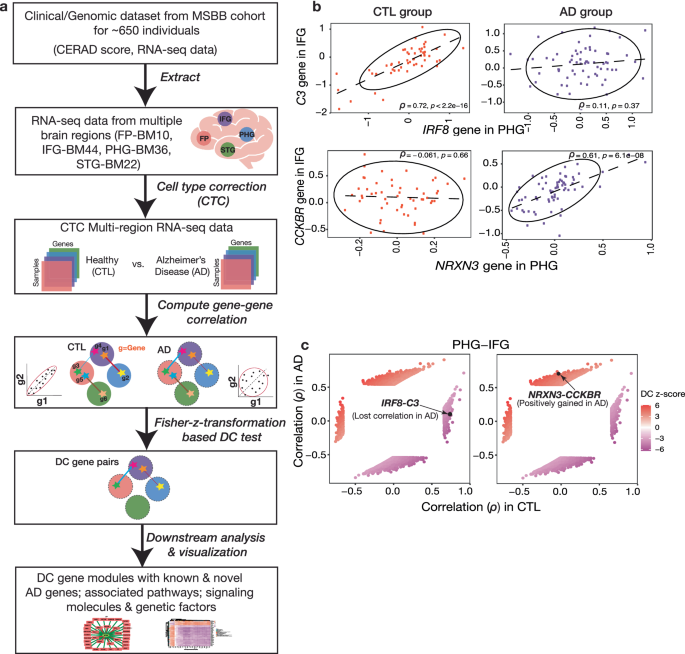
Alzheimer’s is known to spread across brain regions. Traditional network analysis is limited to constructing networks within a brain region and misses out on the molecular (gene-level) understanding of the communication between brain regions. To study gene pair dysregulation in the disease condition across tissues, we developed this framework called InterTissueDC (see Fig 1) designed to answer the question - What are the gene pairs across brain regions whose coupling, as captured in their correlation strength, varies across a set of (postmortem) brain samples?
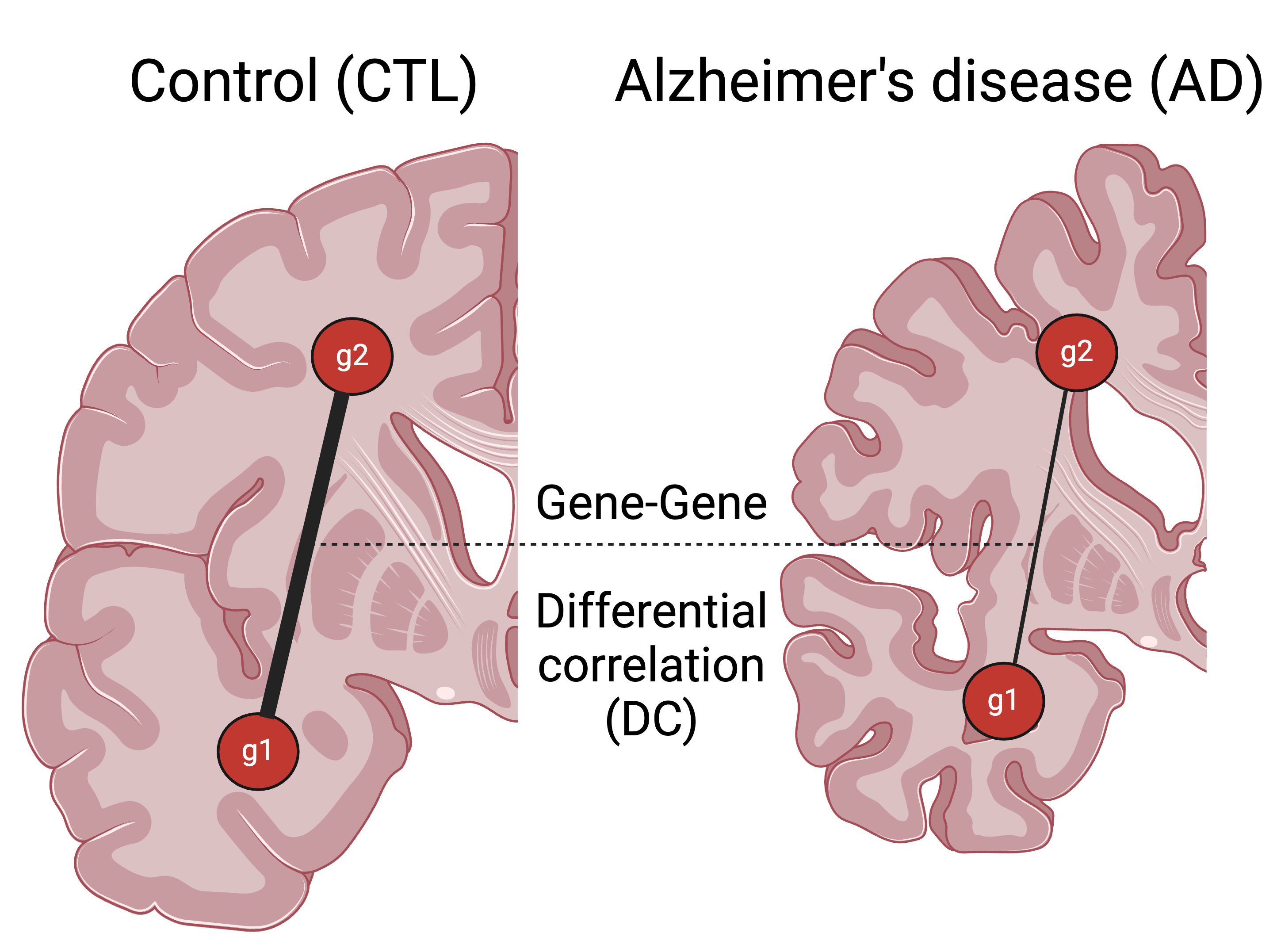
What are the main takeaways from this study?
Between-region insights: We used this framework to study differentially correlated (DC) gene pairs across 4 brain regions sequenced in the Mount Sinai Brain Bank study. We studied the properties of the networks and underlying biological processes across all 6 (4 choose 2) brain region combinations.
A previous study by Wang et al. 2016 ranked 19 brain regions based on their relevance to AD pathology. In our analysis, we found that the most AD-relevant brain region pair had the highest DC dysregulation. Our framework, InterTissueDC, manages to capture this trend among all 6 brain region pairs, with more AD-relevant brain region pairs showing higher DC dysregulation. This confirms that we are capturing AD-relevant biology.
A natural question to ask at this point is, do differential correlation and differential expression capture the same set of genes? To answer this question, we looked at the overlap of DC genes between regions and differential expression (DE) genes in each region. Only 1-9% of DE genes overlap with DC genes, which suggests that DE does not drive DC. So, our analysis reveals disease biology that cannot be uncovered by looking at one region alone.
Unique regional dysregulation patterns among brain regions: Interestingly, we find that different brain regions use different genes for inter-brain region communication, with only 7–21% of DC genes overlapping between different brain regions. Next, we looked at the gene pairs used by one brain region with the other three brain regions. We find that only 20–32% of these DC genes overlap. So, each brain region uses a different gene profile to communicate with another brain region.
Potential Therapeutic Targets: Downstream hub gene analysis on the DC network revealed master dysregulation hubs in AD, including the transcription factor ZKSCAN1, which was previously nominated as a candidate causal gene in myeloid cells. Modules identified among the DC networks were enriched for known AD pathways, such as synaptic signalling and endocytosis.
To enable reproducibility, we have posted the source code and documentation for reproducing the results published in the paper on GitHub: https://github.com/BIRDSgroup/InterTissueDC.
How did the pandemic affect your work?
Funnily enough, MN, SM, and I have never been in the same room together. All of our discussions and preliminary analyses were performed over Zoom (see Fig 2) during the peak of the pandemic. Personally, it helped me through the pandemic, keeping me occupied at a time when everything around the world was uncertain.
What were the venues of publicity for the work? Was it difficult to get the work published?
We presented versions of this work at the CSHL Development & 3D Modeling of the Human Brain 2019 meeting, the Alzheimer's Association International Conference (AAIC) 2022 conference, and several internal and departmental meetings. We are grateful for the valuable insights and feedback we received along the way. Our work was preprinted in 2022, but it took us 2 years to eventually get it published, facing certain rejections along the way. We are glad that our perseverance paid off when our study was finally accepted by npj Systems Biology and Applications.
What are the future directions and limitations? What are the therapeutic implications?
Currently, we are working with Prof. Marcus Seldin and his group at UC Irvine to explore the effects of selected hub genes identified in our analysis in microglia and oligodendrocyte cell lines. We are excited to see what we find here! These experiments will also help validate the main premise of our study, which is that the communication between brain regions often manifests itself as a correlation between the involved genes. Since correlation could arise from various confounding factors besides inter-brain-region signalling/communication, it is important to experimentally test the hypotheses generated from our study.
A technical limitation of our study is the use of bulk RNA-sequencing data, which confounds cell-type-specific contributions. To account for this, we performed cellular deconvolution and adjusted for cell-type proportions, but the ideal solution will be to use spatially resolved single-cell/nuclei RNA-sequencing data. Our framework can be adapted to spatially resolved single-nucleus RNA-seq data to infer cell-type-specific DC networks and identify key dysregulation hubs within specific cell types.
Another limitation of our study is the challenge of obtaining multi-region bulk RNA-sequencing data for anatomically identical regions across various cohorts, which hinders our ability to replicate our results with diverse datasets. So, to show replication, we utilized RNA-sequencing data from anatomically nearby brain regions that were sequenced using an older technology, namely microarrays.
We hope between-region analyses are widely adopted in future research. We need to explore how genes from one brain region affect another and how inter-brain-region communication leads to disease progression. Our research not only sheds light on the intricate gene interactions that occur in Alzheimer’s but also demonstrates the critical importance of considering multiple brain regions in our quest to understand and combat this debilitating disease. As we continue to push the boundaries of what is possible in neurodegenerative disease research, we remain hopeful that our findings will pave the way for effective treatments in the future.
Follow the Topic
-
npj Systems Biology and Applications

An online Open Access journal dedicated to publishing the premier research that takes a systems-oriented approach and encourages studies that integrate, or aid the integration of, data, analyses and insight from molecules to organisms and broader systems.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Systems mechanobiology
Publishing Model: Open Access
Deadline: Mar 28, 2026
Fostering Cross-Disciplinary Modeling in Biology and Medicine
Publishing Model: Open Access
Deadline: May 15, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in