Isolation of mitochondria-derived mitovesicles and subpopulations of microvesicles and exosomes from brain tissues
Published in Protocols & Methods

Extracellular vesicles (EVs) are nanometer-sized vesicles that are constitutively released into the extracellular space by all cell types (Figure 1), including, but not restricted to, bacteria, plant cells, human cell lines, and primary neurons.
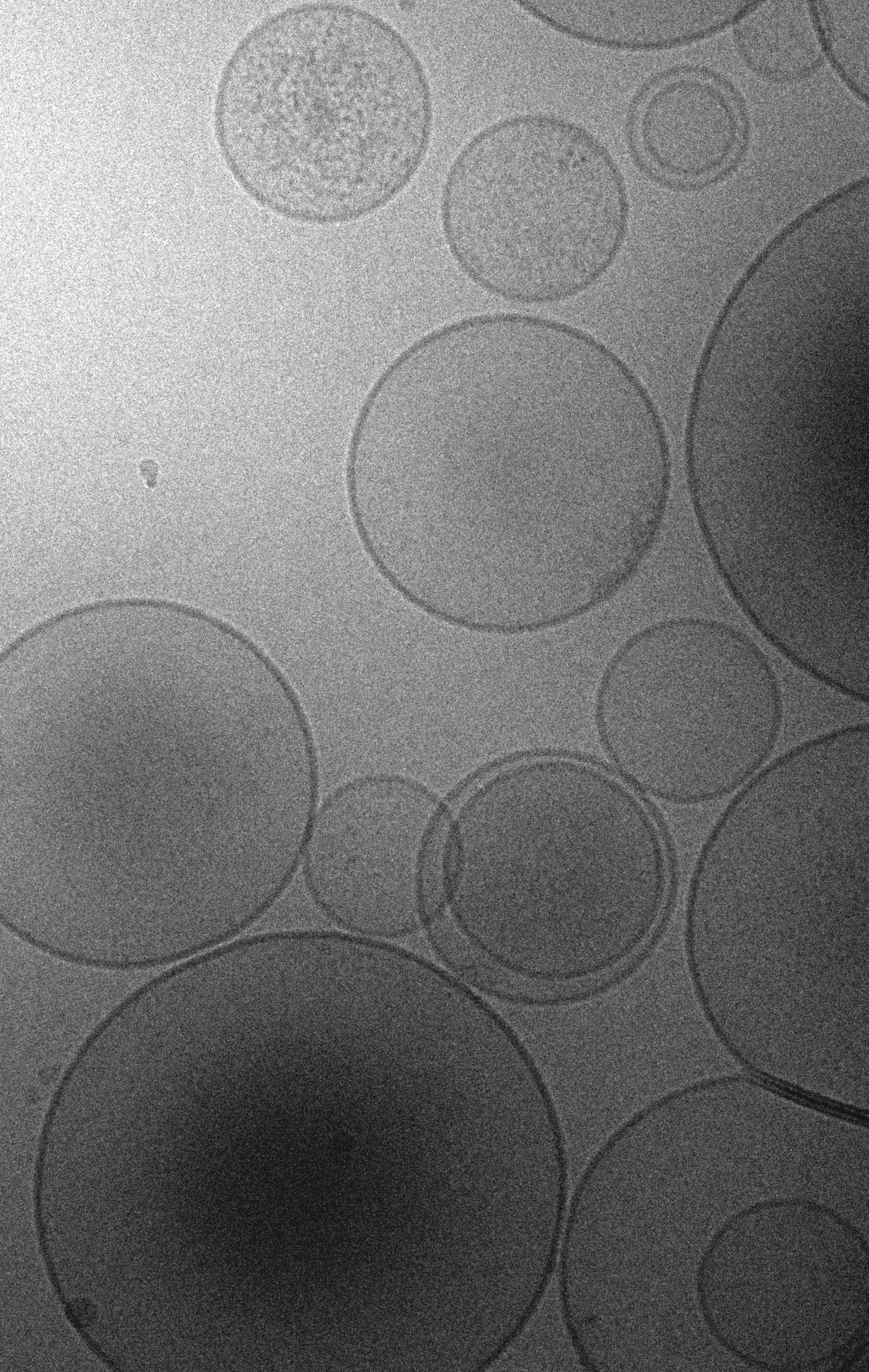
Figure 1. Cryogenic electron microscopy photomicrograph of EVs isolated from brain tissue using the method described in D’Acunzo et al., 2022. Note the presence of small granular EVs (exosomes), larger electron-lucent EVs (microvesicles), and double-membraned EVs (mitovesicles).
By an historical point of view, the journey of EV research has been multi-paced, depending on the methodological tools available at each time period. The first paper to mention the possibility that a “variety of minute breakdown products of the blood corpuscles” could be co-responsible for coagulation was published in 1946 (Chargaff and West, 1946), while the first experimental, electron microscopy (EM)-based description of these platelet-derived corpuscles came more than 20 years later, under the suggestive name of platelet dust (Wolf, 1967). Since those seminal works, studies involving EVs have been scarce until mid-80s, when EVs secreted from reticulocytes were first described, a differential ultracentrifugation approach for EV purification was defined, and the term exosome used for the first time (Johnstone et al., 1987). However, starting from the early 2000s the impact of EVs in the scientific community has been growing exponentially, and rather explosively in the last ten years, with more than 7,000 papers published in 2021 alone mentioning at least once the EV-related terms exosomes, microvesicles, microparticles, or extracellular vesicles (Bazzan et al., 2021).
Why such a tremendously high interest in EVs? The short answer is that EVs are versatile, stable in the body, contain important information on their cell of origin, and can be used as diagnostic tools and as novel therapeutic vectors. EV secretion is constitutive, which means that they can be isolated from any biological fluid (plasma, CSF, urine, semen, saliva, interstitial fluid, amniotic fluid, breast milk, etc.) and in vitro from the culture medium of any cell of interest, from E. coli to HeLa cells. Through EV secretion, cells eliminate undesired material and at the same time transfer molecules from one cell to the other, modulating the biological activity of target cells. This has changed our perspective on the normal physiology of an organism: EVs originating from one cell type can perturb the homeostasis of another cell close by or even at distal sites, and potentially in different organs, a feature that once was considered a prerogative of paracrine factors (growth factors, chemokines, etc.) and hormones. Furthermore, EV cargo and levels can change in case of disease, sometimes even before the symptoms manifest. A consequence of these characteristics is that the analyses of EV constituents can be used for diagnostic purposes, or for follow-up testing to monitor the recovery status of a patient. Lastly, natural EVs are valuable and relatively unexplored platforms to deliver therapeutic biological macromolecules (proteins, miRNA, lipids, etc.) to specific targets. For instance, liposomes (which are proteinless artificial EVs) are routinely used as a system for DNA/RNA transfection in vitro and are precious tools to deliver exogenous mRNAs to recipient organisms in vivo, as in the case of the Pfizer’s and Moderna’s COVID-19 vaccines. Natural EVs are more complex and heterogeneous than liposomes and carry proteins on their surface that are expressed in the respective cell of origin. This implies that EVs can have the machinery to be endocytosed by certain cell types and not others (and, as such, have a greater cell tropism when compared to liposomes), making them attractive tools to deliver molecules in a cell-type specific fashion.
For all these reasons, the attention posed on EVs worldwide is very high, and the potential of EVs in such a variety of applications is just starting to be unveiled. Nevertheless, up to 10 years ago all data found in the literature originated exclusively from biofluids or from in vitro studies. The lack of a method to isolate EVs from the extracellular space of tissues hampered our knowledge of the paracrine role of EVs in physiology and disease in vivo. This was particularly challenging for us, as the major focus of research in our laboratory has been the changes that occur in the brain during aging, as well as in neurodevelopmental and neurodegenerative disorders, and the differences between females and males. Therefore, in order to include in our investigation the roles of EVs in the brain, both physiological and pathological, we developed a novel method to isolate EVs from the interstitial fluid of the brain, initially published in 2012 (Pérez-González et al., 2012) and in more detail in 2017 (Pérez-González et al., 2017). We used this method for the analyses of brain EVs during aging (Kim et al., 2022), in patients with neurodegenerative diseases, such as Alzheimer’s disease (Pérez-González et al., 2020), in individuals with Down syndrome (Gauthier*, Pérez-González* et al., 2017; D’Acunzo et al., 2019), and in subjects carrying the apolipoprotein E4 allele, the greatest genetic risk factor for Alzheimer’s disease (Peng et al., 2019).
Our original isolation method guarantees clean EV preparations but, at the same time, relies on a low-resolution sucrose density gradient as part of the EV purification steps which fails to separate EV subpopulations. The last ten years of research revealed that EVs are heterogeneous and encompass vesicles with different intracellular origins, functions, and molecular markers. It is conceivable that each EV subpopulation has a specific biological role and effect within the body, likely a consequence of the intracellular source and the components it transports. Therefore, we have continuously worked to improve our original protocol, refining the resolution of the gradient to better fractionate known and novel brain EVs (Figure 2). The culmination of our efforts led to a separation technique that generates eight EV fractions, a method that we recently characterized (D’Acunzo et al., 2021) and described in great detail in our last manuscript published in Nature Protocols (D’Acunzo et al., 2022). We used this novel method for the analyses of EVs in the brain chronically exposed to cocaine (Barreto*, D’Acunzo* et al., 2022), in Down syndrome (D’Acunzo et al., 2021), and in spinocerebellar ataxia (Zhang et al., 2021).
* = authors contributed equally
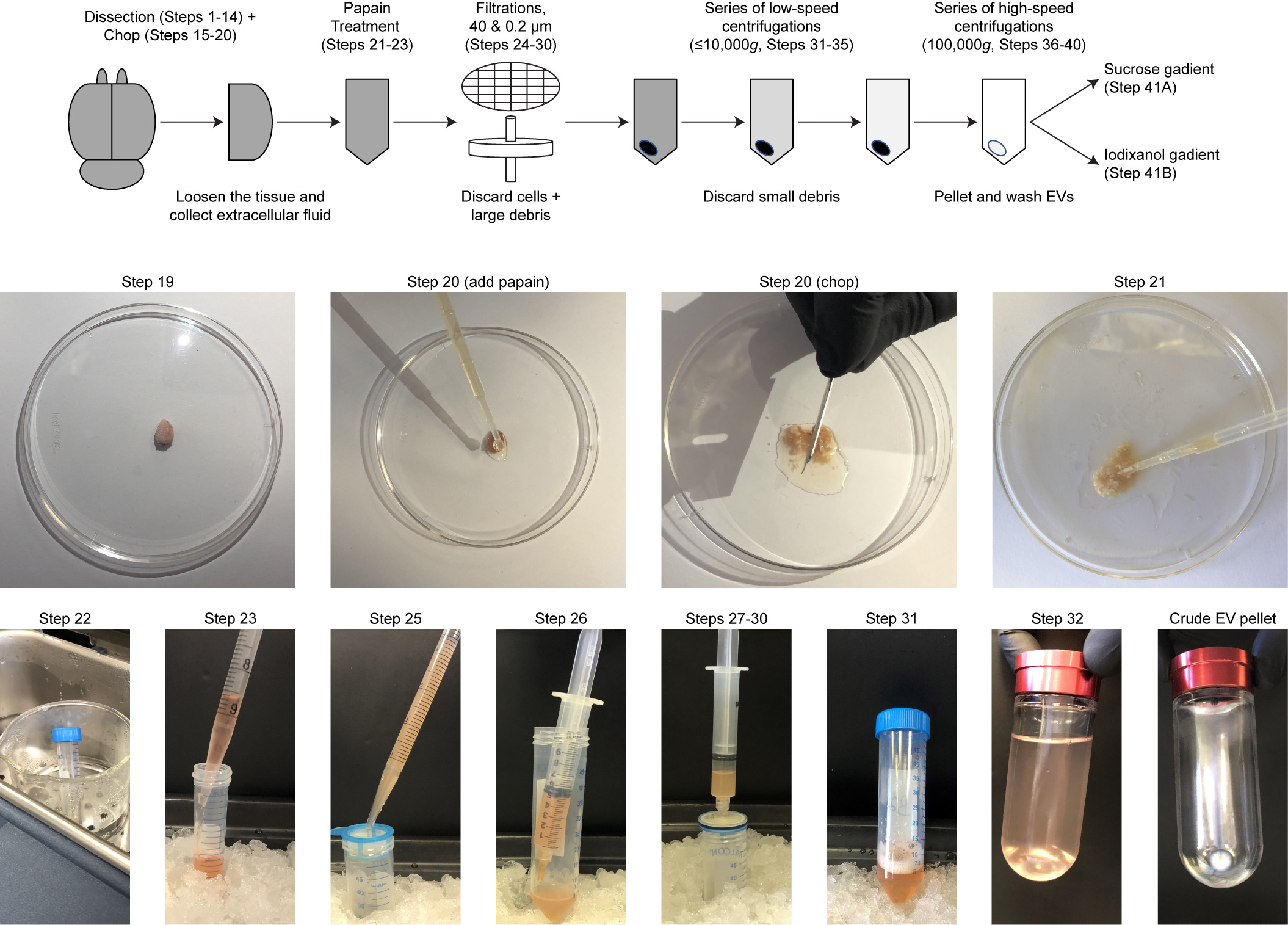
Figure 2. Overview of the first steps of brain EV isolation. The full, detailed protocol is available in D'Acunzo et al., 2022.
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in