IT’S ALL A MATTER OF TIMING: VIRUS REPLICATION AND CIRCADIAN RHYTHMS.
Published in Microbiology
Alan Zhuang, Peter Balfe and Jane A McKeating
The story began with a small cohort of hepatitis C virus (HCV) infected patients undergoing liver transplantation, where we noticed a difference in the kinetics of viral replication in the newly transplanted liver that associated with the time of surgery; where transplantation in the morning associated with increased viral replication kinetics1. Other parameters previously reported to influence viral replication in the post-transplant setting, such as donor age, cold-ischemia time and length of surgery did not associate with viral rebound. We immediately thought of the circadian clock and the knowledge that 20% of genes in the liver are circadian regulated provided the impetus to identify the molecular pathways underlying these clinical data.
TIMED OPENING OF THE GATE.
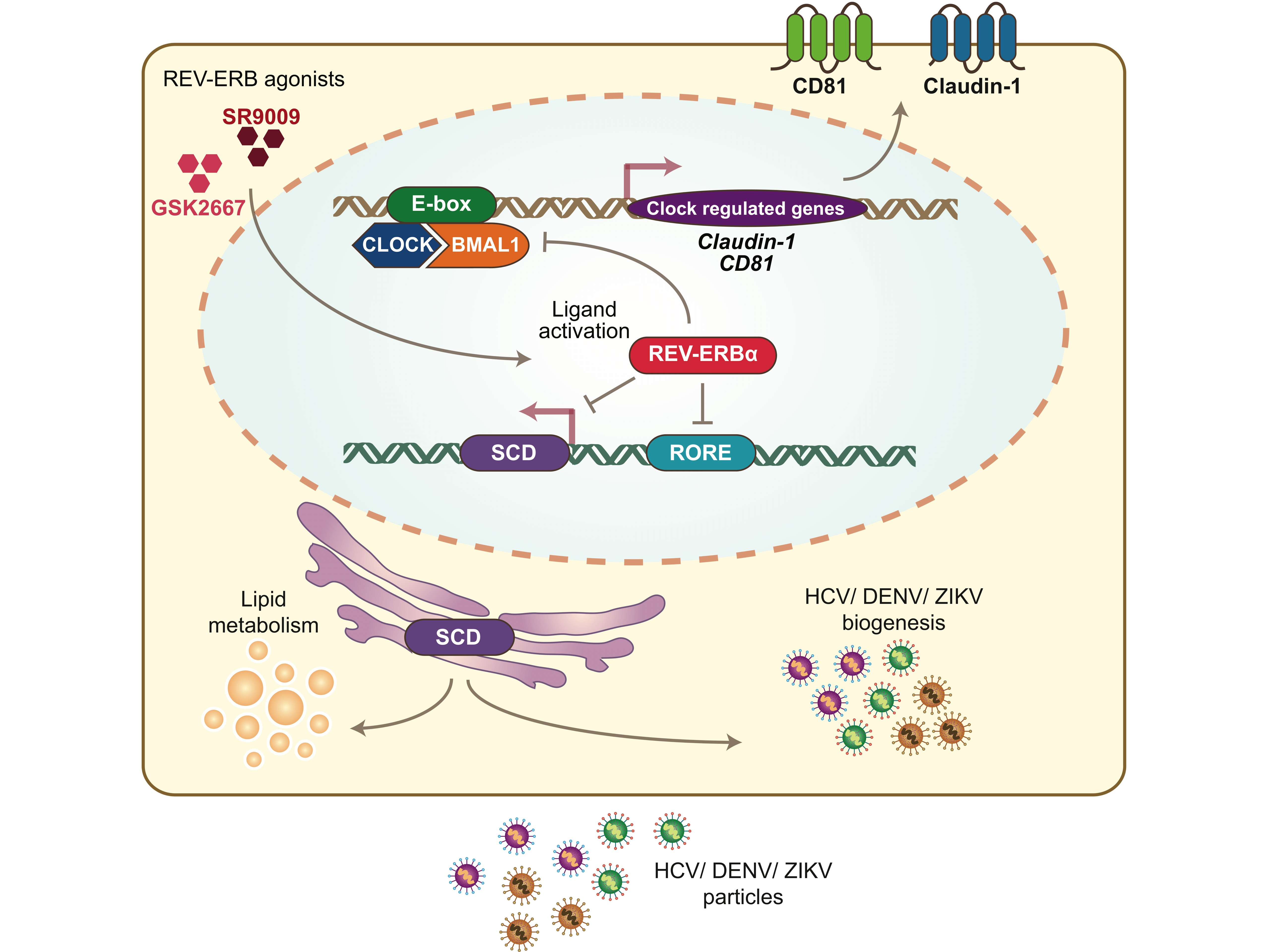
Since our group had been studying the receptors that define HCV entry into the liver for many years, we were interested to know if their expression was circadian regulated. Three of the four essential receptors, CD81, claudin-1 and occludin showed a circadian pattern of transcription in synchronized cells and HCV infection tracked this pattern. Knockout of the circadian transcription factor Bmal1 resulted in a marked down-regulation of CD81 and claudin-1 and reduced infection, not only for HCV but also for the other flaviviruses dengue and Zika, establishing the link between our observations and the circadian clock. https://www.nature.com/articles/s41467-019-08299-7
LIPID METABOLISM AND FLAVIVIRUSES.
It is now well recognized that the circadian transcriptional repressor Rev-Erb regulates hepatic lipid and cholesterol metabolism – key pathways for the genesis and maintenance of the membranous vesicles essential for RNA virus replication and particle assembly. We found that pharmacological activation of Rev-Erb inhibited HCV, dengue and Zika virus infections. Whole-transcriptome microarray analysis of Rev-Erb agonist treated cells confirmed that fatty acid synthesis pathways were severely perturbed, with the major lipid mediator Stearoyl-CoA Desaturase (SCD) identified as a key factor. In addition to its influence on HCV, dengue and Zika virus replication, SCD is required for the formation of viral replication compartments or ‘replication factories’ in West Nile, Human Immunodeficiency Virus and Respiratory Syncytial viruses, extending the significance of our observations to other human pathogenic viruses.
IS BMAL1 PRO- OR ANTI-VIRAL?
Our finding that knocking out Bmal1reduced HCV, dengue and Zika virus replication contrasts with Edgar et al2 study that reported a more severe infection of herpes simplex virus 1 and Influenza A in Bmal1KO mice, highlighting different roles of Bmal1 in the life cycle of these viruses. Our simple model system focused on the role of Bmal1 and Rev-Erb in regulating flavivirus entry and RNA genome replication in the absence of an adaptive immune response. Since the liver is an immune privileged site, one may hypothesize that the influence of Bmal1 on host metabolism may outweigh its effect on the immune response. Future studies on circadian regulation of innate immune activation in response to these viral infections will be of great interest. Exploiting the rhythms of a host cell are great in theory for the virus, but the influence of other rhythms at the organism level may over-ride these benefits. We may have evolved our smart time keeping of host immunity and cellular pathogen susceptibility to minimize the chances of infection.
CAN VIRUSES PERTURB THE CLOCK?
We are only just uncovering the complex interplay between viruses and the clock and one obvious question is whether viral infections can perturb host clock machinery. And if so, what are the down-stream consequences? The report of bio-informatic algorithms to identify cycling genes in clinical material, independent of time-of-day information3, now enables studies to ascertain the effects of viral infection on clock regulated genes. Preliminary results in our laboratory show a perturbation of key clock genes in hepatitis B and C virus associated liver cancer, in agreement with reports associating the circadian clock and cancer metabolism4. Collectively, these studies highlight new chronopharmacological strategies for treating viral infections.
REFERENCES.
1 Zhuang, X., Lai, A. G., McKeating, J. A., Rowe, I. & Balfe, P. Daytime variation in hepatitis C virus replication kinetics following liver transplant. Wellcome Open Res3, 96, doi:10.12688/wellcomeopenres.14696.1 (2018).
2 Edgar, R. S.et al.Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proceedings of the National Academy of Sciences of the United States of America113, 10085-10090, doi:10.1073/pnas.1601895113 (2016).
3 Ruben, M. D.et al.A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med10, doi:10.1126/scitranslmed.aat8806 (2018).
4 Masri, S. & Sassone-Corsi, P. The emerging link between cancer, metabolism, and circadian rhythms. Nat Med24, 1795-1803, doi:10.1038/s41591-018-0271-8 (2018).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in