Unlike most metabolites that are broadly present across cell types, itaconate is unique in that it is mostly produced in innate immune cells in activation-specific manner. Upon stimulation by pathogen- or damage-associated molecular patterns (PAMPs or DAMPs), myeloid cells induce Irg1 (Acod1) expression more than a hundred-fold, leading to the intracellular accumulation of millimolar levels of itaconate. Our lab and others have shown that itaconate plays an important role in TCA cycle remodeling in activating macrophages by inhibiting succinate dehydrogenase (SDH).
In 2016, our lab was the first to identify itaconate as an immunoregulatory metabolite with mild anti-inflammatory effects. Using Irg1-deficient macrophages, we showed that endogenous itaconate moderately suppressed IL-1β secretion in conventional NLRP3 inflammasome activation1. The idea that an immune cell-specific metabolite could modulate immune responses, even modestly, was very intriguing and exciting for the field of immunometabolism which was in the initial expensive phase at that time.
Due to the initially weak phenotypes seen in Irg1 knockout macrophages (macrophages lacking Irg1 and itaconate), the field began using esterified itaconate derivatives like dimethyl itaconate (DI) and 4-octyl itaconate (4-OI), which showed strong inhibition of inflammasome activation and IL-6 production. These were assumed to be membrane-permeable mimics of itaconate. In our own work, we took a dual approach—genetic and pharmacologic—using Irg1-deficient mice and DI, and observed consistent results. We later used the same strategy in our studies on the ATF3 and NRF2 pathways2, where we were the first to describe endogenous itaconate as a mild electrophile.
Yet, the discrepancy in the strength of action between itaconate and its derivatized forms was bothersome to us, and we realized that aggressive popularization of octyl-itaconate as suitable proxy to study itaconate (and even putting the “itaconate” in the title of the papers working exclusively with 4-OI) was misleading and very damaging to the field. In response, we conducted a direct comparison of endogenous itaconate, DI, and 4-OI, analyzing their metabolic, immunological, and electrophilic properties3. Our results were definitive: only endogenous itaconate or Irg1-deficiency should be used to study its biology. The most striking discrepancy involved type I interferon responses—while native itaconate boosted type I IFN following LPS stimulation, derivatives like 4-OI suppressed it. Sadly, even now many labs continue to use and publish 4OI in their studies and refer to it as itaconate research, following the misleading precedents set by some labs4.
Our observation of a strong interferon phenotype with itaconate marked the beginning of a new research direction in the lab.
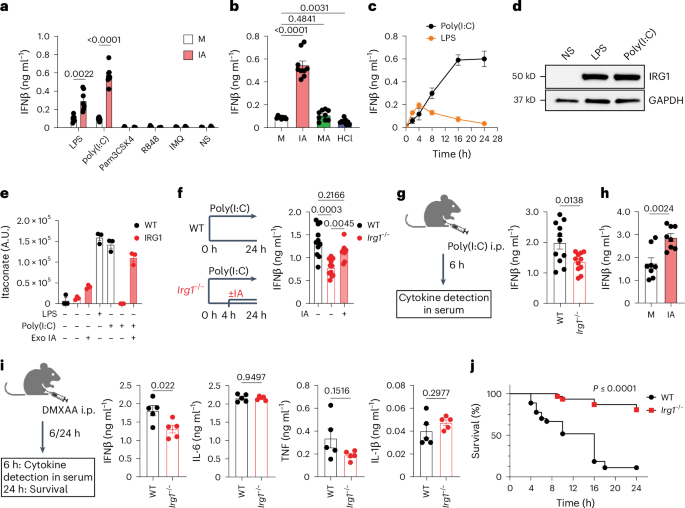
In Swain et al. (2020), we found that endogenous itaconate boosted type I IFN even with LPS, though the effect was subtle, likely because LPS-stimulated BMDMs are not potent IFN producers (well known fact in the field). When we moved beyond LPS to other activation models, we observed striking results—endogenous itaconate clearly enhanced interferon production both in vitro and in vivo5, suggesting a potent immunoenhancing role. This contrasted with the widespread assumption that itaconate is immunosuppressive, including observations reported in our own work. For example, the first truly strong in vitro phenotype for Irg1-deficient macrophages was role of itaconate to establish of late inflammasome tolerance described by us in 20216, a phenotype consistent with an anti-inflammatory role itaconate.
Strong but seemingly contradicting phenotypes – what an exciting, and yet confusing, place to be it was!
With robust immunoregulatory phenotypes in hand, we began probing mechanisms. Given earlier findings that itaconate modifies proteins post-translationally, we initially focused on protein itaconation as a potential cause for the observed IFNβ and inflammasome effects. However, this led to repeated dead ends. Even got us to a point when dropping the project was on the table. We ultimately found that previously described mechanisms—SDH inhibition, NRF2 activation, and cysteine modification—were not the key drivers.
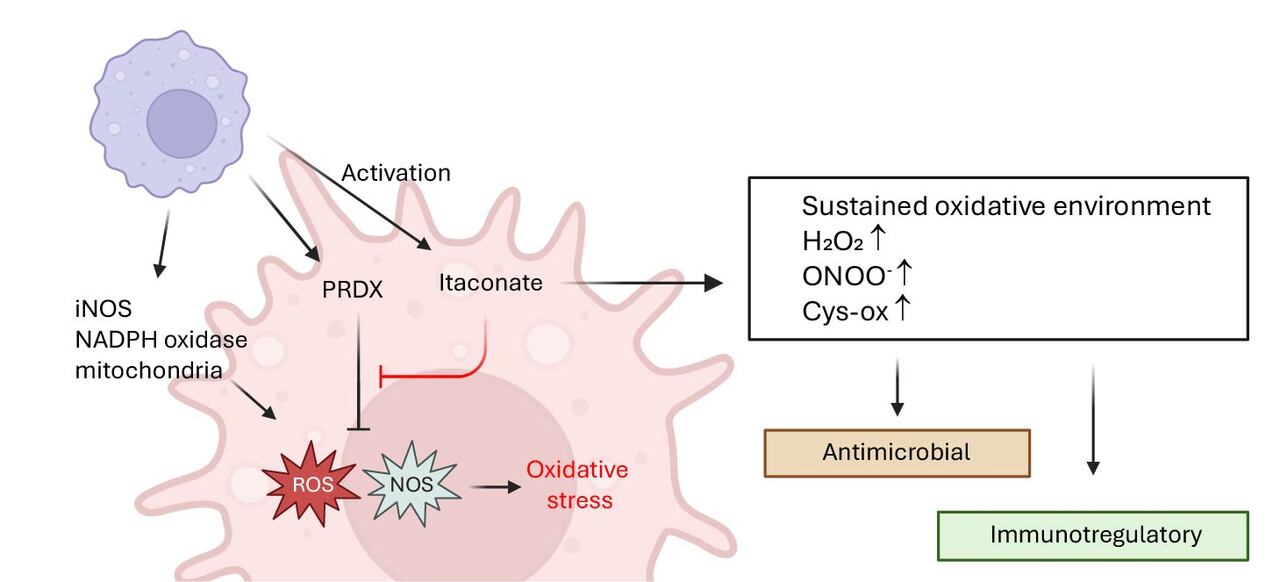
The breakthrough came when we discovered that itaconate modulates mitochondrial hydrogen peroxide, leading to enhanced ROS production that drives IFNβ expression. Investigating further, we identified Peroxiredoxin 5 (PRDX5), a mitochondrial antioxidant enzyme, as a direct target. Itaconate non-covalently inhibits PRDX5, and this inhibition alone was sufficient to drive the interferon boost. We validated this using 2-methyl succinic acid, a novel itaconate mimetic that inhibits PRDX5 but is non-electrophilic and does not inhibit SDH. Yet it reproduced both the type I IFN boost and inflammasome suppression. Around the same time, another study linked SDH inhibition to IFNβ induction (2024), but our data show that this mechanism is not essential for itaconate. While SDH inhibition can support interferon production, it requires concomitant ROS signaling—evident in our experiments with NAC and mitoTempo.
We uncover completely new avenue for itaconate research, “cool” you might say. But why is this really important? Our work provides not only new strong in vitro and in vivo phenotypes and mechanism for immunoregulatory action of itaconate but also provides major insight into evolutionary raison d’etre for connection between itaconate and immune responses. Indeed, itaconate production upon immune stimuli is conserved in innate-like cells in a wide range of species. It gets upregulated in response to multiple stimuli, not only bacterial-associated signals. Why? It can’t be just about inflammasome or even interferon in response to specific stimuli. In fact, the most conserved mode of antimicrobial action by innate immune cells is reactive oxygen/nitrogen species. Our work shows that itaconate inhibits natural antioxidant response in the activating macrophages thus enhancing the action of oxidative species (see Figure). Indeed, it would be counterproductive to produce ROS/RNS with one hand and then suppress it with the other, and that’s where itaconate resolves the conundrum without changing major regulatory circuitry in the cells.
- Lampropoulou, V. et al. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab 24, 158–66 (2016).
- Bambouskova, M. et al. Electrophilic properties of itaconate and derivatives regulate the IκBζ–ATF3 inflammatory axis. Nature 556, 501 (2018).
- Swain, A. et al. Comparative evaluation of itaconate and its derivatives reveals divergent inflammasome and type I interferon regulation in macrophages. Nature Metabolism 2020 2:7 2, 594–602 (2020).
- Mills, E. L. et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117 (2018).
- Paulenda, T. et al. Itaconate modulates immune responses via inhibition of peroxiredoxin 5. Nat Metab (2025) doi:10.1038/s42255-025-01275-0.
- Bambouskova, M. et al. Itaconate confers tolerance to late NLRP3 inflammasome activation. Cell Rep 34, (2021).
Follow the Topic
-
Nature Metabolism

This journal publishes work from across all fields of metabolism research that significantly advances our understanding of metabolic and homeostatic processes in a cellular or broader physiological context, from fundamental cell biology to basic biomedical and translational research.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
Fascinating story! Appreciate the great efforts from you "cool" guys who guided the direction of itaconate research, one of if not THE most significant discoveries in immunometabolism area in the past decade.
one of the remaining questions is, how to cope with the appearing paradox that itaconate can enhance the action of oxidative species, yet inhibit NLRP3 activity on BMDMs, given the main stream belief that mtROS is a trigger of NLRP3 activity.
Perplexing yet fascinating, beauty of science!