Laromestrocel: Stem Cells for Alzheimer's Disease?
Published in Bioengineering & Biotechnology, Neuroscience, and Cell & Molecular Biology
Alzheimer’s disease (AD) is a devastating and progressive neurocognitive disorder, the leading cause of dementia, and a major cause of mortality that will affect up to 13 million people in the US alone by 20501. Although strides have been made recently in the treatment of one of the world’s most fearsome diseases, even the best available options are only partly effective and carry significant risks2. The most serious side effects are known as ARIA—amyloid related imaging abnormalities, due to swelling or hemorrhaging in the brain—which occurs in around a quarter of treated patients. Therefore, other treatments, either stand-alone or that can be combined with existing treatments, are urgently needed.
Infusions of cells known as MSCs (mesenchymal stem cells; also known as medicinal signaling cells) represent an emerging new class of therapy for diverse, difficult-to-treat diseases. Recently, the US Food and Drug Administration (FDA) approved an MSC product called remestemcel-L for steroid-refractory graft versus host disease (GVHD) in children. MSCs can be used as an off-the-shelf treatment because they are immunoprivileged, which in turn enables them to be administered to patients without significant risks of immune reactions or other complications3.
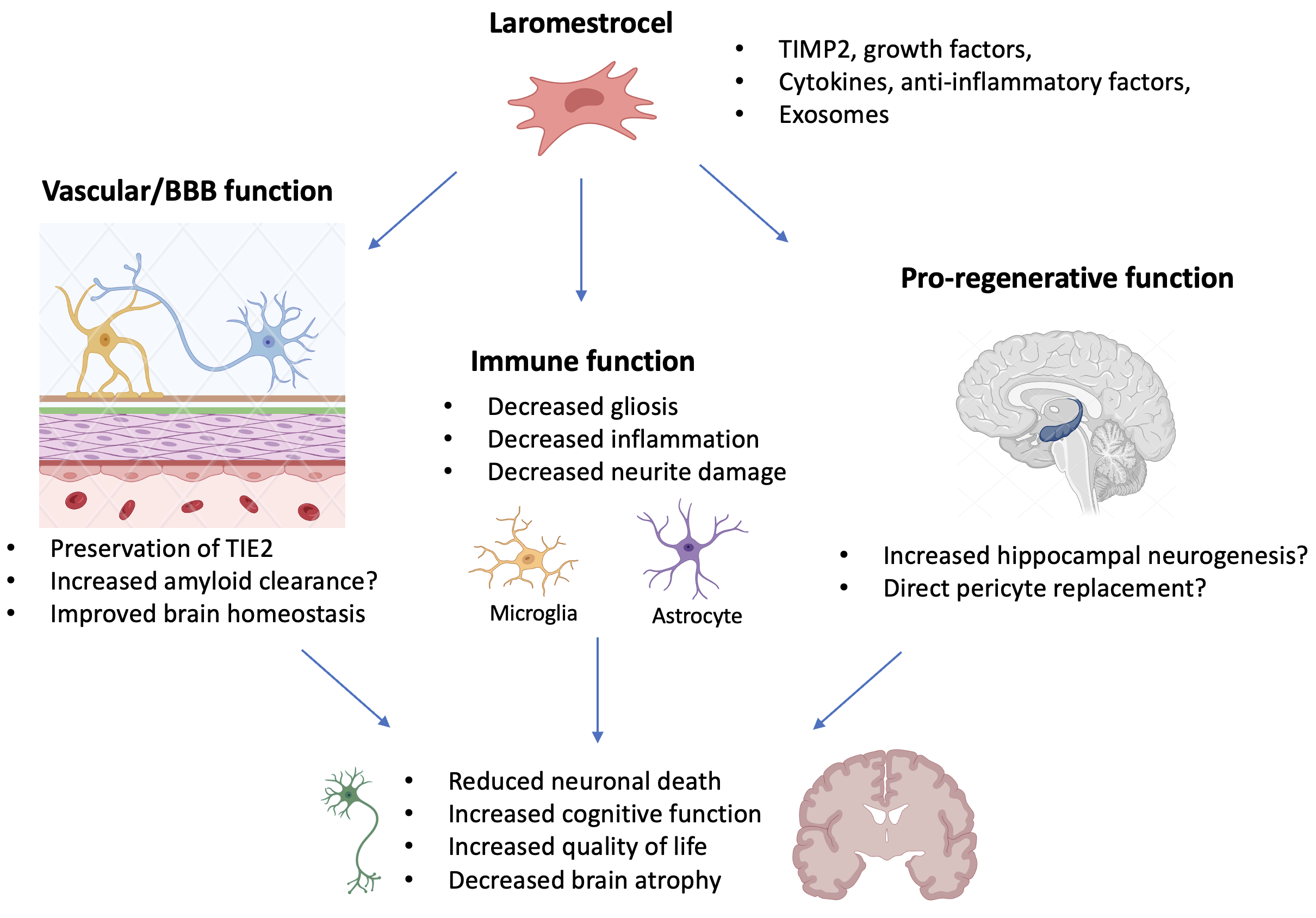
The concept of treating AD with laromestrocel stems from its pro-vascular and anti-inflammatory properties. At the core of the pathophysiology of AD are: 1) damaging neuroinflammation driven at least in part by the extracellular amyloid accumulation in the brain and consequent gliosis, 2) impairment of the blood brain barrier (BBB) and other deficiencies in cerebral blood perfusion, and 3) Tauopathy resulting in the formation of damaging intracellular neurofibrillary tangles within neurons. In combination, these factors cause neuronal loss and lead to general brain atrophy beginning with regions critical to memory formation4. There is interplay between these core factors. For example, a dysfunctional BBB impairs amyloid export from the brain, exacerbating amyloid deposition and in turn neuroinflammatory responses5. Conversely, amyloid accumulation can directly damage the BBB. Animal studies with murine MSCs in an AD model have indicated that treatment reduces microglial activation and potentially causes beneficial changes in Tau phosphorylation6, or might even stimulate neurogenesis (the hippocampus is described to have neurogenic potential even in human adults7) though the precise mechanisms of such effects are unclear.
The CLEAR MIND study
The CLEAR MIND study8 represents the first promising human data for slowing the progression of AD using a stem cell therapy. The treatment intervention was an intravenous infusion of laromestrocel (Lomecel-BTM) either once (25 million cells; 25M; N=12), or four monthly infusions (25M; N=13 or 100M; N=11), and the trial employed a randomized, double-blind, placebo-controlled design. These three treatment groups were compared with placebo patients who received identical-appearing sham infusions (N=12). All enrolled patients met diagnostic criteria for mild AD. The trial duration was 39 weeks, and over that time it showed that the treatments, which included both single and up to four monthly infusions, were well tolerated, with no safety issues identified, no infusion related reactions, and no ARIA detected.
The efficacy results of the study fall into two primary domains: clinical testing and brain volumetry by magnetic resonance imaging (MRI). Clinical tests included well-established measures of cognition, function, activities of daily living, and quality of life. Despite the small study size, the results showed promising efficacy signals and a dose-response effect, particularly in cognition and function, as measured by CDR-SB, and in activities of daily living as observed by caregivers. Clinically meaningful differences compared with placebo were observed in two cognitive assessments: the mini mental state exam (MMSE) and the Montreal cognitive assessment (MoCA). These clinical findings were supported by MRI results, which documented a slowing of brain atrophy both globally and in regions affected by the disease, notably including the hippocampus (a principal brain region responsible for memory), temporal and frontal lobes, thalamus, and lateral ventricles, as compared with placebo. While the present trial was of relatively short duration for an AD study, we speculate that extending the treatment duration and assessment periods could reveal even greater impact to disease progression.
Mechanistically, these findings raise the exciting hypothesis that laromestrocel may be neuroprotective, but how exactly? The study further used exploratory techniques to assess whether laromestrocel could positively affect neuroinflammation or vascular performance. Using a technique called diffusion tensor imaging (DTI), which measures the structural constraints to water diffusion in the brain, we found that neuroinflammation in the cingulate cortex, (an early AD-affected brain region) exhibited a time-dependent worsening in the placebo group that did not occur in patients who received laromestrocel. Additionally, we found serum biomarker evidence consistent with improved vascular and inflammatory pathway (TIE2) integrity with laromestrocel treatment compared with placebo. While there are details of the mechanism of action of laromestrocel that remain to be unraveled (Figure 1), these data support a potential benefit of treatment and provide human data consistent with pro-vascular and anti-inflammatory functions. To follow-up on these exciting findings, we are planning additional research to assess for effects on the core AD-related biomarkers (amyloid, pTau, NfL, and GFAP) and to further examine cerebral blood flow and BBB health. Together with larger clinical studies, these planned mechanistic studies will further shed light on how laromestrocel exerts its effects.
In summary, this study represents a significant advancement in understanding the potential of stem cells in treating AD. The results suggest that laromestrocel may help patients maintain independence from caregiver assistance for a longer period (as measured by ADCS-ADL) and slow cognitive decline, particularly in orientation and working memory (MMSE, MoCA). Administered intravenously over 40 minutes, laromestrocel was well tolerated, with no safety concerns observed, reinforcing its favorable safety profile demonstrated in prior clinical trials across different conditions9,10. The present study thus supports further development of laromestrocel as a potential therapy to slow AD progression. Due to its potential anti-inflammatory and pro-vascular effects, together with the lack of ARIA, laromestrocel could become a stand-alone treatment for AD, or may augment anti-amyloid immunotherapies, potentially providing added benefit to patients.
References
- 2024 Alzheimer's disease facts and figures. Alzheimers Dement 20, 3708-3821 (2024).
- Cummings, J., Osse, A.M.L., Cammann, D., Powell, J. & Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer's Disease. BioDrugs 38, 5-22 (2024).
- Bagno, L.L., Salerno, A.G., Balkan, W. & Hare, J.M. Mechanism of Action of Mesenchymal Stem Cells (MSCs): impact of delivery method. Expert Opin Biol Ther 22, 449-463 (2022).
- Scheltens, P., et al. Alzheimer's disease. Lancet 397, 1577-1590 (2021).
- Greenberg, S.M., et al. Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways. Nat Rev Neurol 16, 30-42 (2020).
- Neves, A.F., et al. Intravenous administration of mesenchymal stem cells reduces Tau phosphorylation and inflammation in the 3xTg-AD mouse model of Alzheimer's disease. Exp Neurol 341, 113706 (2021).
- Gage, F.H. New neurons are born in the adult human brain. Nat Med 31, 356-357 (2025).
- Rash BG, R.K., Agafonova N, Naioti E, McClain-Moss L, Zainul Z, Varnado B, Peterson K, Brown M, Leal T, Kopcho S, Carballosa R, Patel P, Brody M, Herskowitz B, Fuquay A, Rodrigues S, Jacobson AF, Leon R, Pfeffer M, Schwartzbard JB, Botbyl J, Oliva AA Jr, Hare JM. Allogeneic mesenchymal stem cell therapy with laromestrocel in mild Alzheimer’s disease: a randomized controlled phase 2a trial. Nat Med (2025).
- Brody, M. et al. Results and insights from a phase I clinical trial of Lomecel-B for Alzheimer’s disease. Alzheimers Dement. 19, 261–273 (2023).
- Tompkins, B. A. et al. Allogeneic mesenchymal stem cells ameliorate aging frailty: a phase ii randomized, double-blind, placebo-controlled clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. 72, 1513–1522 (2017).
Follow the Topic
-
Nature Medicine

This journal encompasses original research ranging from new concepts in human biology and disease pathogenesis to new therapeutic modalities and drug development, to all phases of clinical work, as well as innovative technologies aimed at improving human health.
Related Collections
With Collections, you can get published faster and increase your visibility.
Stem cell-derived therapies
Publishing Model: Hybrid
Deadline: Mar 26, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in