Linking nanotechnology and microbiome for cancer therapy
Published in Bioengineering & Biotechnology
Using mice models for human cancer research remains the mainstream strategy for preclinical studies. To minimize the species difference between mice and humans, scientists are developing clinically-relevant or humanized mouse models for cancer research. In one of our recent studies, which aimed to use more clinically-relevant cancer models, an orthotopic colorectal cancer model was established by injecting tumor cells directly into the mouse caecum. Surprisingly, we noticed that the orthotopic colorectal tumor demonstrated a different response to anti-PD-L1 treatment compared to subcutaneous tumors established from the same cell line. While many micro-environment differences exist between orthotopic and subcutaneous tumors, we asked whether factors arising from the gut influence the orthotopic tumor due to the close proximity to the colon. To test this, we used polymyxin B to modulate the composition of the microbiome, and discovered that T cell infiltration into the orthotopic tumor was recovered alongside a reduction of inflammatory signals. Furthermore, we focused on the lipopolysaccharide (LPS)/Toll-like receptor 4 (TLR4) pathway, designed nanoformulations for tumor-specific LPS clearance and TLR4 blockade, and established the role of gut-derived LPS in modulating the immune microenvironment in our orthotopic colorectal cancer model [1].
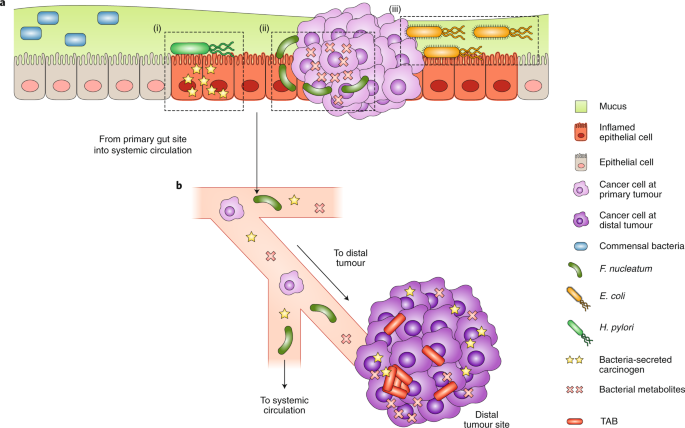
Revisiting this study, we think many opportunities at the microbiome/cancer interface exist. The role of the microbiome extends to host immunity and cancer therapy as well. In fact, metabolites from the microbiome serve as signals for these complex interactions. These metabolites may be synthesized de novo by gut microbes, produced by these bugs from our dietary components, or even produced by our body and then biochemically modified by our microbiome. For example, primary bile acids are secreted by our hepatocytes into the small intestine for assisting food nutrients absorption; when primary bile acids enter the large intestine, they are converted into secondary bile acids by the microbes through deconjugation or dehydroxylation. Primary and secondary bile acids have different receptors, and a recent study reported that the gut microbiome affected liver cancer therapy through modulating the balance between primary and secondary bile acids [2]. Although small differences in the chemical structures between primary bile acids and secondary acids exist, they have completely different effects on our body systems – our body uses these small differences as signals for programming host responses.
As these signals are identified, more and more targets for cancer therapy are arising. Our article focuses on discussing how nanotechnologies can be used to modulate these signals, the receptors and downstream pathways for these signals, or even the microbes producing these signals. Through controlling these interactions with nanotechnologies, we posit that new approaches for precision cancer therapy will emerge. These key opportunities and past efforts are highlighted in our review paper in Nature Nanotechnology - “Nanotechnology Intervention of the Microbiome for Cancer Therapy” [3].
References
[1] Song W., et al. Trapping of lipopolysaccharide to promote immunotherapy against colorectal cancer and attenuate liver metastasis. Advanced Materials 30, 1805007 (2018).
[2] Ma C., et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
[3] Song W., Anselmo A. C. & Huang L. Nanotechnology intervention of the microbiome for cancer therapy. Nature Nanotechnology 14, 1093-1103 (2019).
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in