Mapping the substrates of human pseudouridine synthases
Published in Cell & Molecular Biology
Ψ is the earliest known RNA modification and one of the most abundant. In humans, 13 PUS enzymes are encoded in the genome. Of these, 12 (including RPUSD1–4, PUS1, PUS3, PUSL1, PUS7, PUS7L, TRUB1, TRUB2, and PUS10) are considered “stand-alone” enzymes acting directly on RNA. The remaining enzyme, DKC1, works together with small nucleolar RNAs to catalyze Ψ modifications in ribosomal and small nuclear RNAs. Although some of these enzymes have been studied in yeast, the specific “writers” responsible for most Ψ sites in human tRNAs have remained unknown.
In recent years, increasing evidence has linked mutations or altered expression of stand-alone PUS enzymes to a wide range of human diseases. Systematically mapping the RNA targets of these stand-alone PUS enzymes is therefore important for understanding their biological roles, and how their disruption might contribute to diseases.
The challenge: link each pseudouridine site to its writer(s)
One of the main difficulties in studying pseudouridine is detecting it accurately, particularly in tRNA. Traditional N-cyclohexyl-N’-(2-morpholinoethyl)carbodiimide methyl-p-toluenesulfonate (CMC)-based detection methods are often noisy and imprecise, while newer bisulfite-based methods struggle to distinguish Ψ located within consecutive and dense uridine regions, which are common in tRNAs.
To overcome these limitations, our group developed 2-bromoacrylamide-assisted cyclization sequencing (BACS). BACS enables accurate Ψ detection at base resolution by directly introducing Ψ-to-C mutations, and outperforms other methods in robustly identifying precise Ψ positions.
When we first applied BACS to human cells, we discovered the Ψ landscape in human tRNAs differs substantially from that previously reported in yeast, reflecting the greater complexity of tRNA and PUS enzymes in humans. These findings motivated us to map which writers were responsible for which Ψ sites in human tRNAs.
The approach: a systematic knockout effort
To address this question, we used CRISPR-Cas9 to individually knock out each PUS enzyme, and then detected the change in Ψ level using BACS. Achieving complete knockouts was essential, as even small amounts of PUS enzyme might have been sufficient to sustain Ψ modifications. Clean knockouts also allowed us to examine potential redundancy between PUS enzymes.
Our first success came with PUS7, TRUB1, and PUS1 knockout HeLa cell lines. Using BACS, we mapped their dependent Ψ sites in both cytosolic and mitochondrial tRNAs, revealing broader and sometimes unexpected substrates. Encouraged by these interesting findings, we collaborated with Mengjie Li from Dr. Parinaz Mehdipour’s group to systematically knock out the remaining nine stand-alone PUS enzymes, and worked closely with Drs Haiqi Xu and Giuseppina Pisignano, combining our strengths in CRISPR design, BACS experiments, and data analysis to ensure the success of the project.
As often happens in science, the process was not straightforward. For instance, we struggled to find a reliable commercial antibody to validate the depletion of PUS7L by western blot, and instead validated the knockout by Sanger sequencing. Some knockout attempts failed in the first round; for others, despite repeated efforts and even switching to another cell line, depletion was impossible, most likely because the enzymes are essential for cell survival. In such cases, we shifted our strategy to obtain efficient knockdown of the enzymes using short hairpin RNAs.
Building a comprehensive map
All these collective efforts eventually paid off, culminating in the most comprehensive Ψ map of human tRNAs to date. For the first time, we identified the in vivo targets for several PUS enzymes, including RPUSD1, RPUSD2, PUS3, PUSL1, PUS7L, PUS10 and TRUB1.
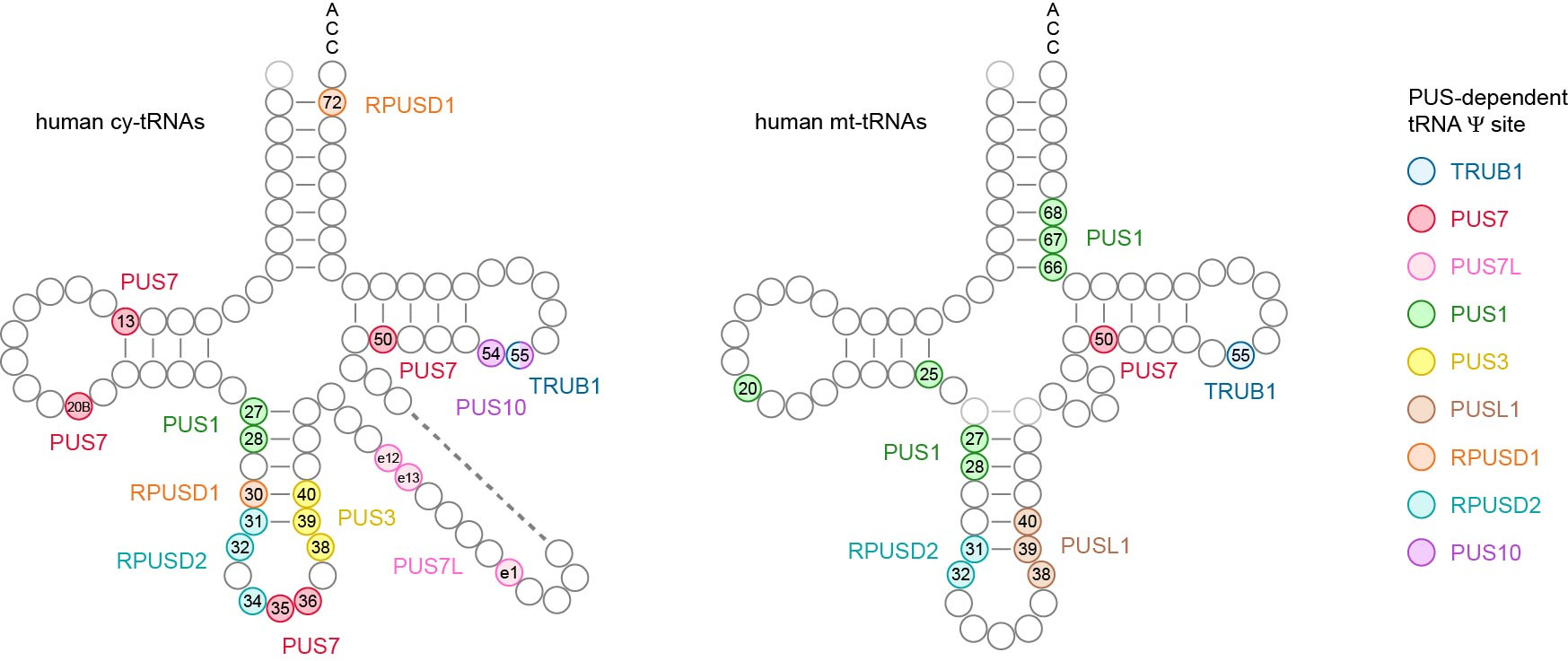
Among our discoveries:
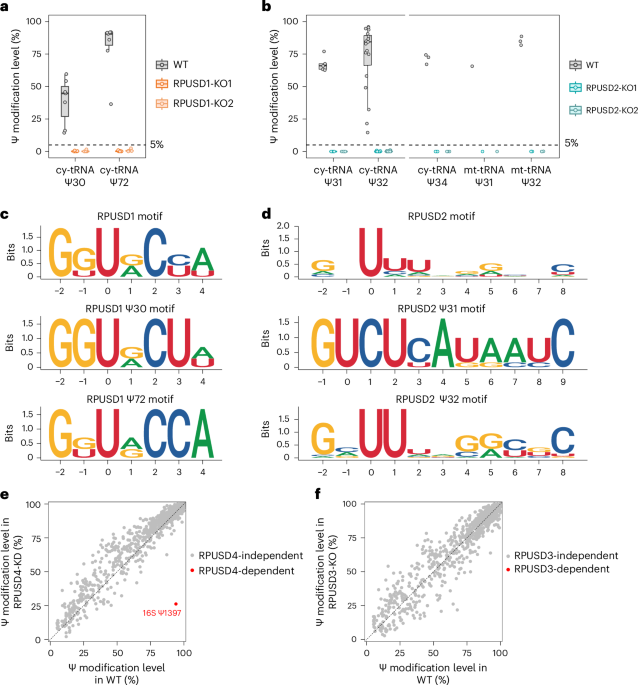
- Despite belonging to the same protein family, RPUSD1 and RPUSD2 display distinct enzymatic activities. RPUSD1 catalyzes Ψ30 and Ψ72 in cytosolic tRNAs, while RPUSD2 modifies not only Ψ31–32 in both cytosolic and mitochondrial tRNAs, but also Ψ34 in the anticodon region of cytosolic tRNAs.
- In contrast to yeast, PUS3 only catalyzes the Ψ38–40 modification in cytosolic tRNAs, while Ψ38–40 in mitochondrial tRNAs is modified by PUSL1.
- PUS7L exhibits unique enzymatic activities within the variable region of cytosolic tRNAs.
- TRUB1 and PUS10 share redundant activity at the highly conserved Ψ55 position in cytosolic tRNAs.
This comprehensive Ψ map highlights the specialized target specificity of human stand-alone PUS enzymes on tRNAs, and offers a valuable resource to aid understanding of the biological functions of Ψ and the diverse roles of PUS enzymes in human cells.
Looking ahead
Why do human cells maintain such a complex and highly specialized network for PUS enzymes to install pseudouridine modifications in tRNAs? Moving forward, we want to understand the consequences of losing specific PUS enzymes under physiological conditions, and in the context of cancers.
This study marks an important step towards illuminating how Ψ RNA modifications, once invisible to us, shape the molecular language of life.
ACKNOWLEDGEMENT. The author of this post is grateful for Dr. Claire Barber and Dr. Chunxiao Song for editing this blog article.
Our paper: Xu, H., Kong, L., Li, M. et al. A comprehensive tRNA pseudouridine map uncovers targets dependent on human stand-alone pseudouridine synthases. Nat Cell Biol (2025). https://doi.org/10.1038/s41556-025-01803-w
Follow the Topic
-
Nature Cell Biology

This journal publishes papers of the highest quality from all areas of cell biology, encouraging those that shed light on the mechanisms underlying fundamental cell biological processes.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in