Metal-free Photoinduced C(sp3)–H/C(sp3)–H Cross-Coupling to access α‑Tertiary Amino Acid Derivatives
Published in Chemistry
The Zheng group has devoted to the design and synthesis of chiral catalysts, nerve drugs, anticancer drugs and other frontier fields, and has achieved a series of innovative results (Chem.Rev. 2018, 118, 7586; ACIE. 2018, 57, 1601; J. Med. Chem. 2019, 62, 6015). Notably, unnatural ATAAs are one of the most abundant moieties in natural products, pharmaceuticals, agrochemicals, and organocatalysts1,2. Replacing natural α‑tertiary amino acids (ATAAs) with unnatural α-amino acid residues could drastically change the solubility, lipophilicity, pharmacokinetics, and physiological properties of the molecules with minor steric alterations. This makes ATAAs versatile building blocks for the synthesis of biologically active molecules. The facile synthesis of unnatural ATAAs using enzymatic or transition metals catalysis has been extensively explored, it still meets many limitations, such as specific reaction types, narrow functional groups, multi-step protocols, and toxic reagents3,4. On the other hand, photocatalysis has witnessed dramatic developments to synthesize ATAAs due to its environmental friendliness, mild conditions and high functional group compatibility5,6. Moreover, considering the cost of photocatalyst and the industrial application, we want to develop a greener and more efficient methodological strategy to synthesize ATAAs. The Zheng group focus on the development of economic and practical (PC- and Metal-free) visible photocatalytic radical reaction for the construction of complex molecules (Selected research works: ACIE. 2019, 58, 14666; Chem. Sci., 2021, 12, 15399; ACIE. 2022, 61, e202210755). In our recent paper published on Nature Communication, we described the application of metal-free CDC reaction for the construction of ATAAs with good regioselectivity under mild condition.
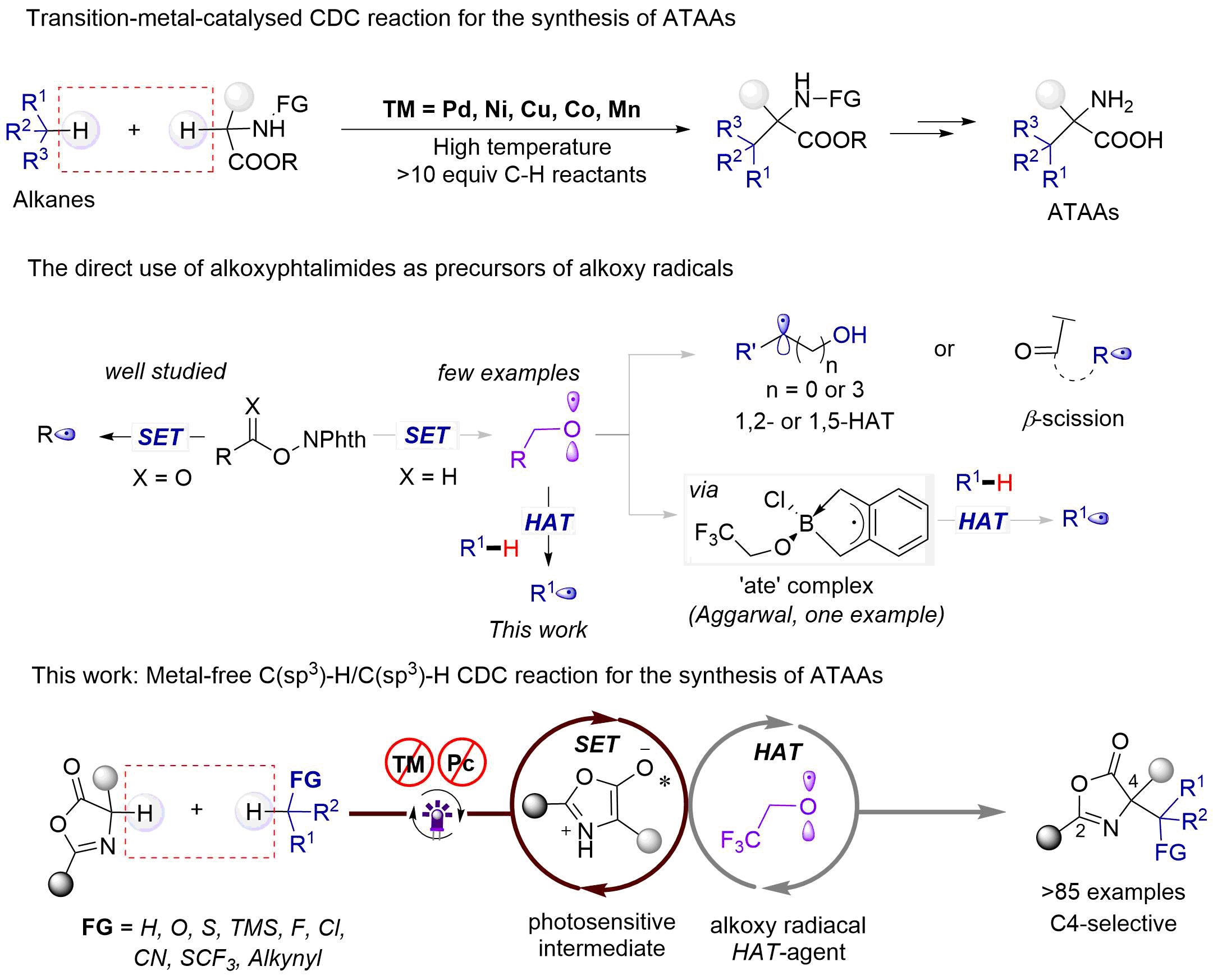
Recently, continuous progress has been made in preparing unnatural ATAAs derivatives by a myriad of synthetic methods. The transition-metal-catalysed (e.g., Pd, Ni, Cu, Co, Mn et al) C(sp3)–H/C(sp3)–H cross dehydrogenative coupling (CDC) reaction represents one of the most reliable approaches towards the formation of ATAAs assisted by chemical oxidant (e.g., DTBP, TBHP et al)7. On the other hand, we recently developed a metal-free method for the synthesis of valuable unnatural α-amino acid derivatives using aliphatic carboxylic acids as alkyl radical precursors with high efficiency and step economy8. The disadvantage is that less compatibility of 3˚ alkyl carboxylic acid substrates and silane substrates containing carboxylic acid. So, stable and commercially available C(sp3)–H feedstock instead of reactive alkyl precursor (carboxylic acids) in these transformations is highly significant because additional chemical steps and purifications are required to prepare the reactive building blocks can be avoided, thereby increasing efficiency. However, utilization of oxazolones in CDC reaction has rarely been explored due to favored dimerization of the azaallyl radical species of oxazolones under oxidizing or metal conditions, along with competitive side reactions9. In addition, it is also challenging to choose the efficient and selective hydrogen atom transfer (HAT) reagent in redox-neutral condition.
In this manuscript, we report a photoinduced metal-free C(sp3)–H/C(sp3)–H cross dehydrogenative coupling (CDC) reaction in redox-neutral condition using a reactive trifluoroethyl oxygen radical (CF3CH2O•) as a hydrogen atom transfer (HAT) reagent. Over 85 unnatural ATAAs with vastly diversified functional groups were synthesized with excellent regioselectivity (C4/C2 >20/1) from abundant hydrocarbon feedstocks under mild conditions (metal-, PC-, and additive-free, ambient temperature). This metal-free strategy provided excellent functional group tolerance and late-stage applicability. Mechanistic studies revealed the reactive trifluoro ethoxy radical act as a HAT agent in the reaction, abstracting a hydrogen atom from hydrocarbon to generate an alkyl radical. We anticipate that this HAT reagent has the potential for widespread adoption in the field of photochemistry.
For details of this work, please see our paper titled “Metal-free Photoinduced C(sp3)–H/C(sp3)–H Cross-Coupling to access α‑Tertiary Amino Acid Derivatives” in Nature Communication.
- Jane, D., Pittaway, K., Sunter, D., Thomas, N. & Watkins, J. New phenylglycine derivatives with potent and selective antagonist activity at presynaptic glutamate receptors in neonatal rat spinal cord. Neuropharmacology 34, 851-856 (1995).
- Toniolo, C., Crisma, M., Formaggio, F. & Peggion, C. Control of peptide conformation by the Thorpe‐Ingold effect (Cα‐tetrasubstitution). Biopolymers 60, 396-419 (2001).
- Wang, J., Liu, X. & Feng, X. Asymmetric strecker reactions. Rev. 111, 6947-6983 (2011).
- Hedges, J. B. & Ryan, K. S. Biosynthetic pathways to nonproteinogenic α-amino acids. Rev. 120, 3161-3209 (2019).
- Blackwell, J. H., Kumar, R. & Gaunt, M. J. Visible-Light-Mediated Carbonyl Alkylative Amination to All-Alkyl α-Tertiary Amino Acid Derivatives. Am. Chem. Soc. 143, 1598-1609 (2021)
- Aguilar Troyano, F. J., Merkens, K., Anwar, K. & Gómez‐Suárez, A. Radical‐Based Synthesis and Modification of Amino Acids. Chem. Int. Ed. 60, 1098-1115 (2021).
- Tsuji, T. et al. α-Amino acid and peptide synthesis using catalytic cross-dehydrogenative coupling. Synth. 1, 304-312 (2022).
- Li, Y. et al. Modular Construction of Unnatural α‐Tertiary Amino Acid Derivatives by Multicomponent Radical Cross‐Couplings. Chem. Int. Ed. 61, e202210755 (2022).
- Tang, S.; Zhang, X.; Sun, J.; Niu, D.; Chruma, J. J. 2-Azaallyl Anions, 2-Azaallyl Cations, 2-Azaallyl Radicals, and Azomethine Ylides. Rev. 118, 10393-10457 (2018).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in