Microwave-assisted synthesis of metal-organic chalcogenolate assemblies as electrocatalysts for syngas production
Published in Chemistry

Amount of anthropogenic carbon dioxide (CO2) has adverse effects on climate. Converting this waste product to value-added chemicals might pave the way for a carbon-neutral future. Syngas (a mixture of hydrogen and carbon monoxide) is a starting material for many of these chemicals. Syngas is mainly produced through 3 different processes which require high temperature/pressure as well as expensive catalysts. Catalysts that can produce syngas at lower temperatures and ambient pressure with high rates are required.
In this study, we explore a relatively new material class, called Metal Organic Chalcogenolate Assemblies (MOCHAs) as catalysts for the electrohemical reduction of CO2. MOCHAs consist of a metal center bound to phenyl chalcogenides (S, Se or Te) in a coordination polymer-like structure. This forms a 2D layer which is stacked on top of others to form a 3D structure. MOCHAs were first introduced in 2002 but gained more attention in 2018 thanks to the extensive studies of Hohman group at University of Connecticut. They can be synthesized using various methods (interfacial, tarnishing). These synthesis methods give rather low product quantities. In our study, we overcome these problems and shorten the synthesis duration from 72 h (in case of tarnishing and interfacial synthesis) to 5 h using microwave-assisted synthesis. We obtain product yield reaching 110mg which allowed us to prepare MOCHA inks to deposit on carbon paper electrodes.
Furthermore, we investigated the catalytic performance of MOCHAs in producing syngas from CO2. MOCHAs delivered a syngas composition of 1:1 (CO:H2) and reached production rates of tens of micromoles per hour.
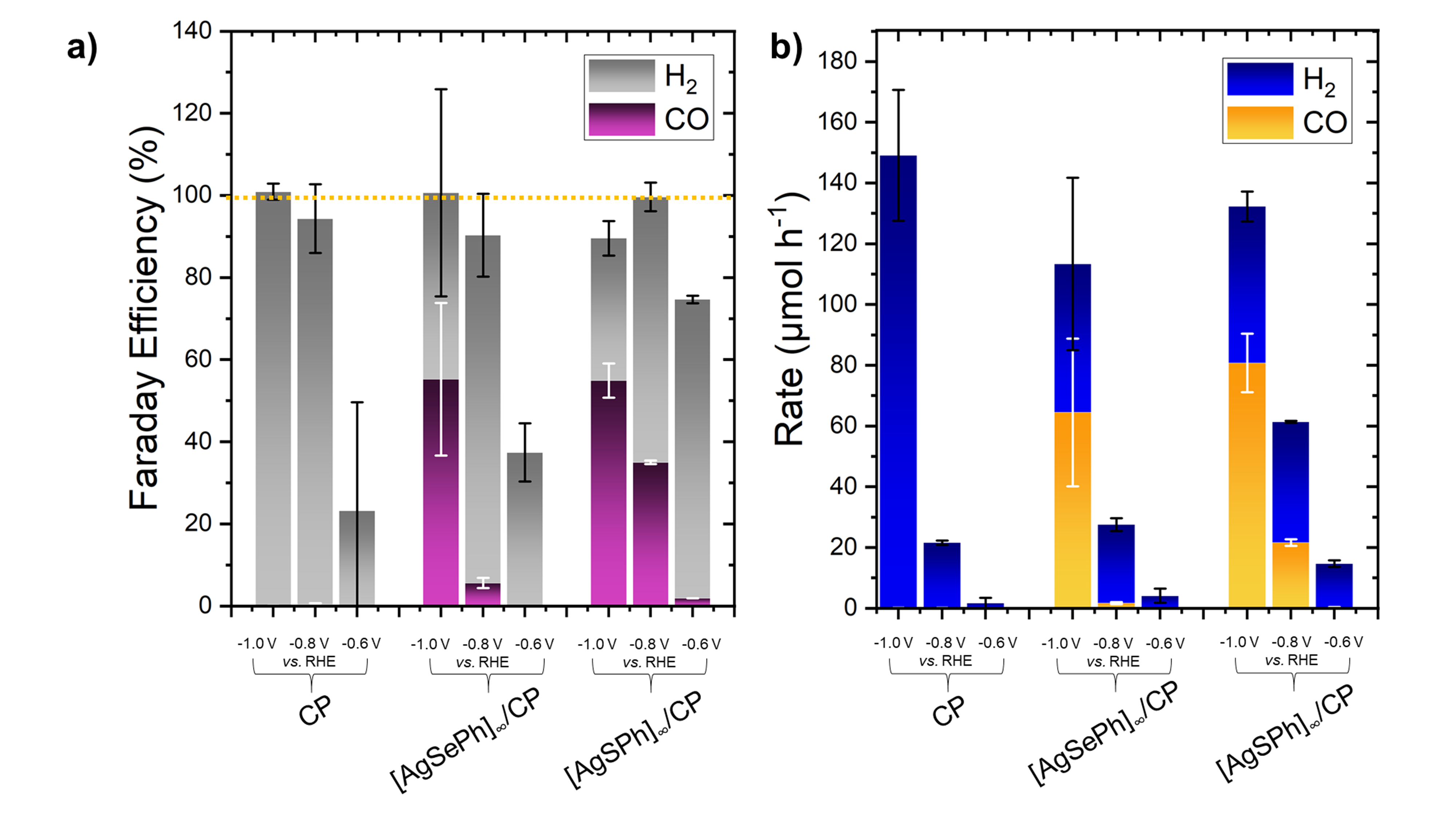
Finally, we tested MOCHAs for their stability in various solvents, temperatures and pH conditions. They show remarkable stability in pristine form as well as post catalysis. Repetitive use of the same electrode shows a ~20% decline in performance after 4 cycles of electrolysis.
We believe our contribution of the new synthesis method will open up the possibilities for further applications of these fascinating materials. In addition, we also showed that the electrocatalytic CO2 reduction community can benefit from such organic-inorganic hybrid approaches.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in