More neurons, less Alzheimer's: from zebrafish to mammals
Published in Neuroscience

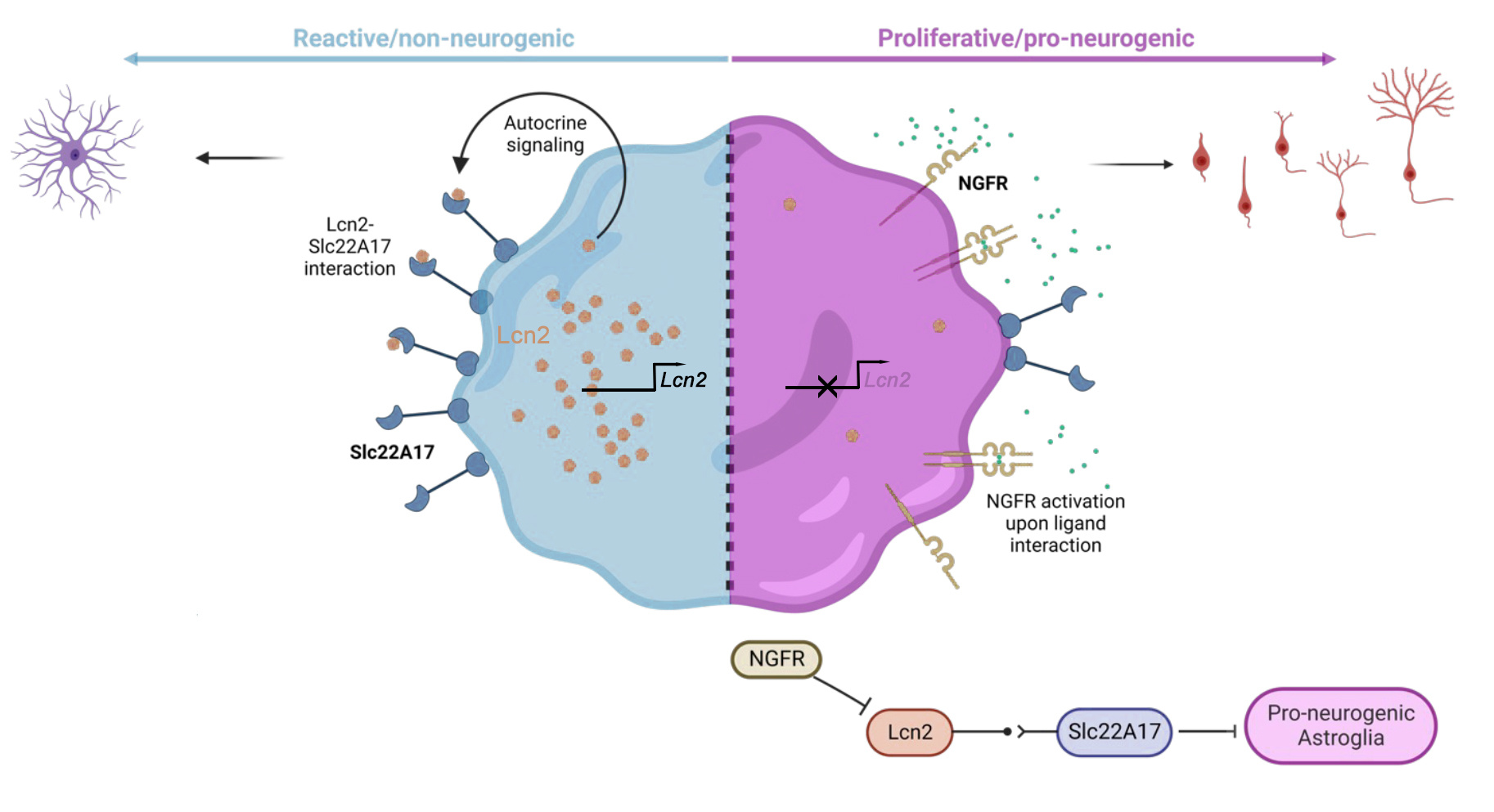
In our recent publication, we uncovered a mechanism related to the brain's ability to generate new neurons, a process called neurogenesis. Several studies showed that this process is reduced in Alzheimer's disease (AD) (e.g., 1). This led us to investigate how we could enhance neurogenesis to potentially counteract the effects of age-related neurodegeneration. Our study focused on astroglial cells, which have the potency to become new neurons, and we explored the role of a receptor called Ngfr (nerve growth factor receptor) in promoting neurogenesis in an AD mouse model. Since we previously identified in zebrafish brain, which is a remarkably regenerative organ even after AD-like neurodegeneration (2), that Ngfr signalling is a determinant of the neuro-regenerative potential and neurogenic capacity of astroglia (3,4), we used this mechanism to address its translational potential in mammalian brains. Our hypothesis was that Ngfr signalling could impose a neuro-regenerative outcome in diseased mammalian brains, and this could ameliorate AD pathology.
We used the zebrafish brain as a model to learn how successful neuro-regeneration could take place after AD-like neurodegeneration and translated this knowledge to mammalian brains
To understand the molecular mechanisms that might kick start the pathology-induced neurogenesis, we used advanced techniques such as single-cell transcriptomics and spatial proteomics as well as 3D human neurogenesis assays and large AD patient cohorts in humans. We found that activating Ngfr signalling in mouse astroglial cells resulted in a remarkable transformation. The cells shifted from a reactive gliotic state, which is associated with disease pathology, to a pro-neurogenic fate, promoting the generation of new neurons. This effect was accompanied by reduced amyloid plaques and Tau phosphorylation, which are characteristic features of AD.
We found that induced neurogenesis through a regenerative mechanisms could reduce the pathological hallmarks of Alzheimer's disease in a mouse model.
To validate our findings, we extended our research to human samples. We analyzed postmortem AD brains and found elevated levels of a protein called Lipocalin-2 (Lcn2), which correlated with reactive gliosis and reduced neurogenesis. In hydrogel-based 3D human neurogenesis assays that we previously developed (5, 6), we confirmed that LCN2 reduced neurogenesis and enhanced reactive gliosis. By blocking the receptor for Lcn2, called Slc22a17, we observed the pro-neurogenic effects similar to Ngfr activation.
Our findings suggest that manipulating the Ngfr/Lcn2 axis could be a potential therapeutic approach for enhancing neurogenesis and alleviating the pathology of AD.
We also compared our results to studies in zebrafish, a species known for its remarkable regenerative abilities. This cross-species comparison provided insights into common downstream effectors of NGFR signaling, including a gene called PFKP, which codes a critical enzyme for energy metabolism and plays a negative role in neurogenesis. These findings suggest that understanding the mechanisms of neuroregeneration in zebrafish could inform novel therapeutic strategies for AD in humans.
Our study highlights the importance of astroglia, the potential precursors of new neurons, in AD pathology. We also identified potential drug targets, such as Slc22a17, and candidate genes, like PFKP, which could be targeted to modulate neurogenesis and potentially mitigate the effects of AD.
By promoting a pro-neurogenic fate in astroglia, we could potentially enhance the brain's ability to cope with neurodegenerative diseases.
Our findings may open up exciting possibilities for developing innovative therapeutic approaches that harness the brain's regenerative potential to combat AD and other neurodegenerative conditions. We also propose that "zebrafish-to-human" approach is a fertile route to determine how we can enhance the resilience of our brains through forming more neurons throughout our lives. We also consider the use of comparative aspects of zebrafish for screening new chemicals to determine novel lead compounds that alter neurogenesis and could be disease modifying as we have recently demonstrated to be a fertile research area (7, 8).
While our study provides valuable insights, there is still much to learn about the complex mechanisms underlying AD and the cognitive implications of promoting neurogenesis in the context of the disease.
References
(1) Moreno-Jimenez, E. P. et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat Med 25, 554-560, doi:10.1038/s41591-019-0375-9 (2019).
(2) Bhattarai, P. et al. IL4/STAT6 signaling activates neural stem cell proliferation and neurogenesis upon Amyloid-β42 aggregation in adult zebrafish brain. Cell Reports 17, 941–948, doi:10.1016/j.celrep.2016.09.075 (2016).
(3) Bhattarai, P. et al. Neuron-glia interaction through Serotonin-BDNF-NGFR axis enables regenerative neurogenesis in Alzheimer’s model of adult zebrafish brain. PLoS Biol 18, e3000585, doi:10.1371/journal.pbio.3000585. (2020).
(4) Cosacak, M. I. et al. Single-Cell Transcriptomics Analyses of Neural Stem Cell Heterogeneity and Contextual Plasticity in a Zebrafish Brain Model of Amyloid Toxicity. Cell Rep 27, 1307-1318 e1303, doi:10.1016/j.celrep.2019.03.090 (2019).
(5) Papadimitriou, C. et al. 3D Culture Method for Alzheimer's Disease Modeling Reveals Interleukin-4 Rescues Abeta42-Induced Loss of Human Neural Stem Cell Plasticity. Dev Cell 46, 85-101 e108, doi:10.1016/j.devcel.2018.06.005 (2018).
(6) Siddiqui T, Celikkaya H, Atasavum ZT, Popova S, Freudenberg U, Werner C, Kizil C. Three-Dimensional Biohybrid StarPEG-Heparin Hydrogel Cultures for Modeling Human Neuronal Development and Alzheimer's Disease Pathology. Methods Mol Biol. 2561:159-170, doi: 10.1007/978-1-0716-2655-9_8 (2023).
(7) Turgutalp, B. et al. Discovery of Potent Cholinesterase Inhibition-Based Multi-Target-Directed Lead Compounds for Synaptoprotection in Alzheimer’s Disease. J Med Chem 65(18):12292-12318, doi: 10.1021/acs.jmedchem.2c01003 (2022).
(8) Bhattarai, P., Turgutalp, B. & Kizil, C. Zebrafish as an Experimental and Preclinical Model for Alzheimer's Disease. ACS Chem Neurosci 13, 2939-2941, doi:10.1021/acschemneuro.2c00583 (2022).
Follow the Topic
-
npj Regenerative Medicine

This journal is an open access, online-only, peer-reviewed journal dedicated to publishing high-quality research on ways to help the human body repair, replace and regenerate damaged tissues and organs.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Cellular and Genetic Tools in Regenerative Medicine
Publishing Model: Open Access
Deadline: Sep 11, 2026
Heart Regeneration and Beyond-Volume II
Publishing Model: Open Access
Deadline: Jun 01, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in