Multiomic single-cell sequencing defines tissue-specific responses in Stevens-Johnson syndrome and toxic epidermal necrolysis
Published in Immunology

Severe skin conditions like Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening reactions leading to death in up to 50% or more of those they affect. They cause the skin and mucous membranes to blister and peel, leading to significant long-term complications like blindness, internal organ, reproductive and mental health complications. In adults these reactions are often triggered by medications, but until now, scientists had little understanding of how the immune system creates such devastating effects.
In a new study published online October 8, 2024 in Nature Communications1, we have uncovered important insights into the interactions between immune cells and skin cells leading to these extreme reactions. By using advanced sequencing techniques at a single-cell level to study the skin and blister fluid of patients with SJS/TEN, we identified the key actor in this process: cytotoxic CD8+ T-cells, a type of immune cell that normally helps protect the body from infections.
What’s intriguing about our discovery is the process by which these CD8+ T cells become activated and deadly. We found that skin cells (keratinocytes) themselves that express a key protein known as human leukocyte antigen (HLA) are able to activate these T-cell assassins. Individuals expressing specific types of HLA proteins are at risk for reactions associated with specific drugs. Once activated, the CD8+ T cells kill the skin cells (keratinocytes) that armed them. Intriguingly, we suggest that keratinocytes lose some of their usual defenses, making them easy targets for the CD8+ T cells. This vicious and self-destructive cycle leads to the devastating and sometimes fatal damage to skin and other epithelial surfaces such as the lining of the mouth, genital tract and eye seen in patients with SJS/TEN.
So what does this mean for people at risk for SJS/TEN?
Although the CD8+ T cells expand and attack after exposure to the specific drug that triggers them, previous work from our group has shown that for certain medications, people with a specific genetic predisposition defined by their HLA type are more likely to develop SJS/TEN. This means that HLA typing could be used to screen individuals before prescribing these drugs; potentially preventing these reactions from occurring in the first place.
Our discovery of specific molecular and cellular signatures that define SJS/TEN as well as the signaling pathways of how cells communicate with each other at a single-cell level opens the door to develop better and more specific strategies for earlier diagnosis, more targeted treatments, and even prevention of SJS/TEN. In essence understanding how the immune system can mistakenly turn against its own skin cells, provides insight into how to stop this process in its tracks, offering hope to prevent death and mitigate harm from this life-threatening disease.
These findings represent a major advance in understanding rare and life-threatening drug reactions like SJS/TEN, and they could lead to personalized approaches that could save lives and prevent long-term harm.
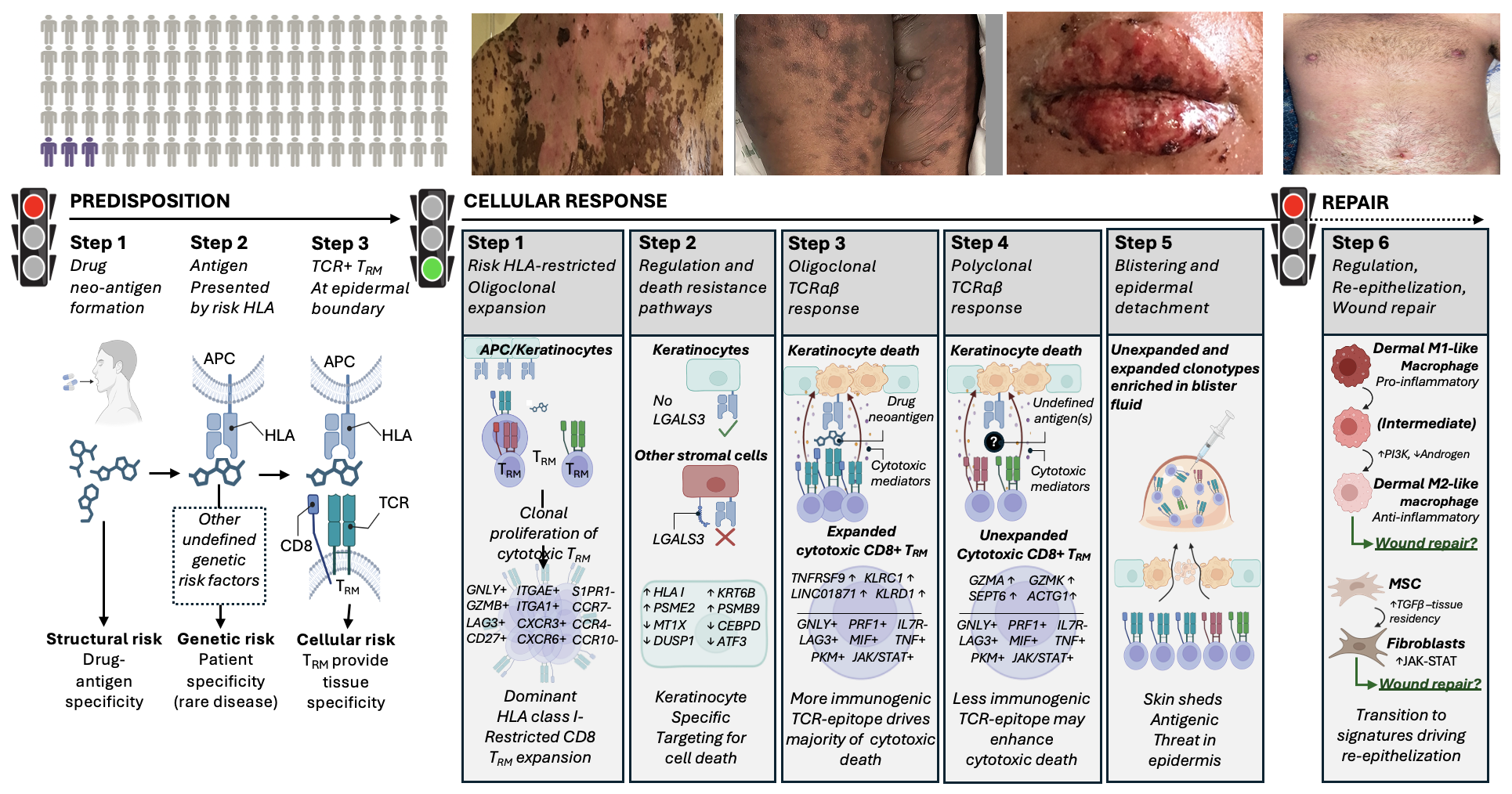
Figure 1: Predisposition: Only the minority of those carrying an HLA risk allele for that drug develop SJS/TEN (2-8%), at risk individuals shown in purple. The risk HLA allele is necessary but not sufficient for the onset of SJS/TEN and other currently undefined genetic risk factors may also contribute to disease predisposition Cellular response: Patients with acute SJS/TEN. Drug-neoantigen is presented by keratinocytes and potentially other stromal cells in the skin by the HLA class I risk allele. Repair: Patient shown in healing stage of SJS/TEN. Pro-inflammatory M1 and intermediate-like macrophages increase PI3K and androgen signalling towards anti-inflammatory populations associated with re-epithelization and repair. Created in BioRender. Phillips, E. (2024) BioRender.com/n10c928. APC, antigen-presenting cell; HLA, human leukocyte antigen; TCR, T-cell receptor; TRM, tissue-resident memory.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in