Myosin 7A is a Miniature Molecular Motor with a Major Mission
Published in Biomedical Research
The inner ear is a sensory organ specialized to detect minute mechanical vibrations such as sound and acceleration. While sensing sound is crucial for animals, detecting sound is not an easy task for terrestrial organisms, because sound waves usually displace air particles by less than one micron. To capture such an extremely minute physical phenomenon, the inner ear has sensory epithelia equipped with mechanosensory hair cells (Fig. 1A). The apical surface of hair cells is covered by brush-like mechanosensors, stereocilia, which bathed in endolymph and deflect sound or acceleration propagated in endolymph as sub-micron mechanical stimuli (Fig. 1B).
Decades of studies on human hereditary hearing loss have shown that normal development and maintenance of stereocilia requires at least four unconventional myosins: myosin IIIA (MYO3A), myosin VI (MYO6), myosin VIIA (MYO7A), and myosin XVA (MYO15A). These myosins are presumed to transport specific stereocilia components as their cargo (i.e., active cargo transport) and anchor them to the actin core of stereocilia. In particular, MYO7A motor activity is required to transport proteins of the mechanoelectrical transduction (MET) machinery. Mice with mutations of MYO7A exhibit profound hearing loss because their stereocilia lack some components of MET machinery. Stereocilia are also severely deformed in these mouse models, indicating that functional MET machinery is indispensable for maintaining the morphology of stereocilia.
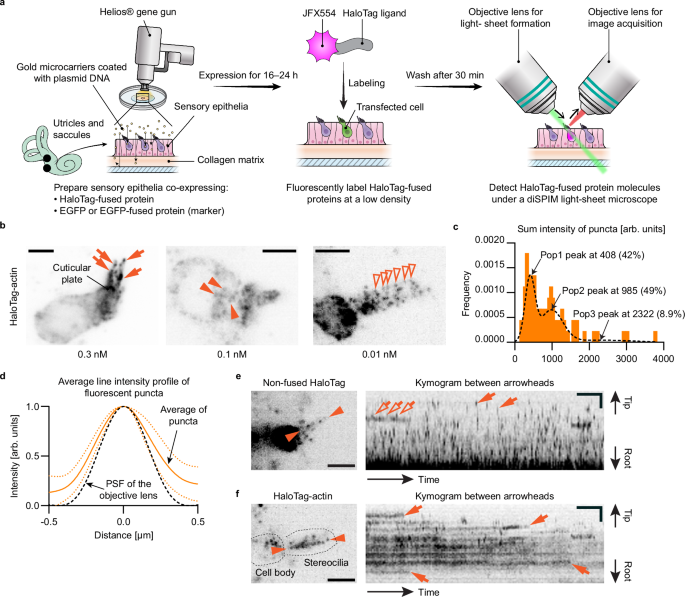
The mechanisms of active cargo transport within stereocilia have been a long-standing question in hearing research. For example, it remains unclear whether MYO7A functions as a monomer like class-I myosins, a dimer like class-V myosins, or a higher-order oligomer like non-muscle class-II myosins. To fill this knowledge gap, we established a novel single-molecule imaging platform, STELLA (Single-Molecule Tracking and Enhanced Localization in Live Aural specimens). STELLA resolves HaloTag-fused single protein molecules in live stereocilia by labeling the HaloTag with fluorescent ligands. Currently, STELLA employs a dual-inverted selective plane illumination microscope (diSPIM) developed by Dr. Hari Shroff (Janelia Research Campus) to achieve a light sheet aligned to stereocilia. HaloTag is labeled with a ligand conjugated with JFX554, one of the brightest organic dyes developed by Dr. Luke Lavis (Janelia Research Campus).
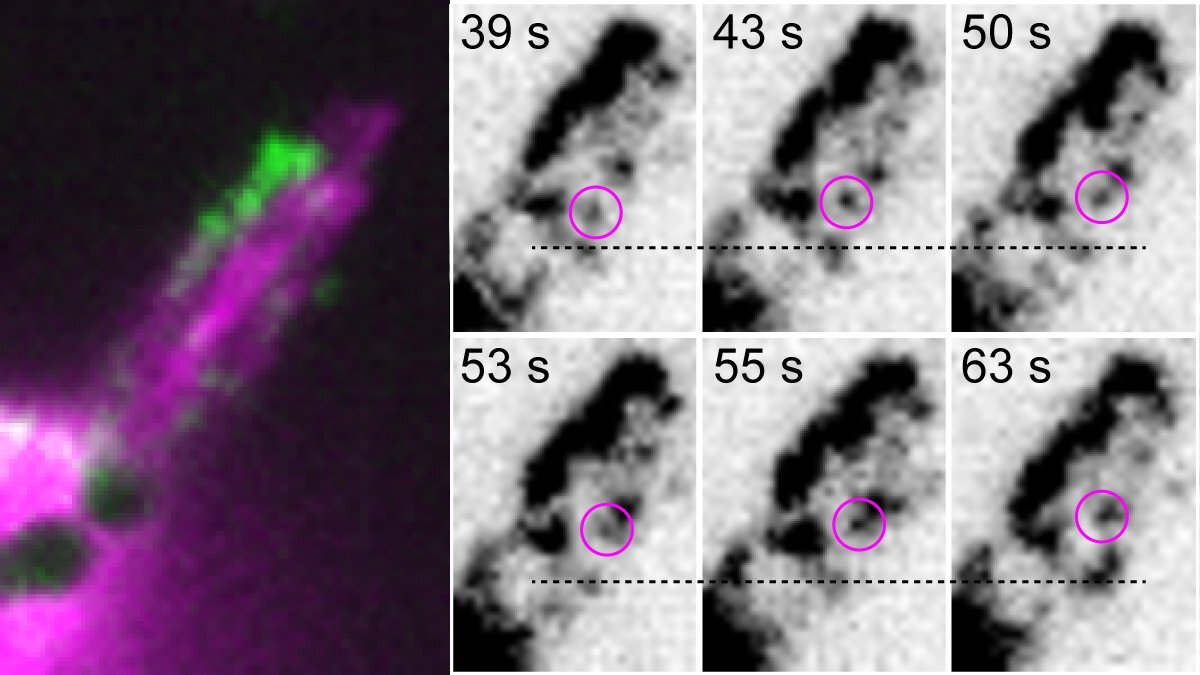
Using this platform, we were able to track individual MYO7A molecules within stereocilia of live murine hair cells (Fig. 2, example shown for dimers, see Movie). We found that MYO7A exhibits processive movement toward stereocilia tips when chemically dimerized or constitutively activated by missense mutations disabling its tail-mediated autoinhibition. From our current data, we posit that MYO7A is activated and dimerized in stereocilia via its cargo, likely one of the components of the MET machinery.
STELLA has opened new avenues to capture real-time dynamics of protein molecules within live hair cells. Such methods will remain essential tools in hearing research, as the intracellular environment of inner ear hair cells is not easily reconstituted by conventional assays using recombinant proteins and cultured cells. Particularly, functional analysis is challenging for proteins that function in structures unique to hair cells (e.g., stereocilia and ribbon synapses) and those rarely expressed in other tissues (e.g., MYO15A and OTOF). STELLA can also be applied to the functional evaluation of genetic variants associated with hearing loss. In combination with in silico prediction tools such as AlphaFold and AlphaMissense, STELLA may help accurately reclassify numerous variants of uncertain significance (VUS) at a level useful for clinical practice. Although many VUS are likely to represent benign polymorphisms, part of them may in fact be pathogenic variants that could mislead clinical decision-making. Currently, more than 120,000 missense VUSs have been reported for hearing loss in the Deadness Variation Database—over 4,000 of them are in MYO7A alone.
The techniques used in STELLA are applicable to other three-dimensional tissues. In addition to promoting hearing research, our approach may facilitate research in other complex systems, such as neuronal layers, retinal pigmented epithelium and photoreceptors of an eye, the intestinal brush border, and invasive cancer.
This research was supported [in part] by the Intramural Research Program of the NIDCD at the National Institutes of Health (NIH). The contributions of the NIH author) are considered Works of the United States Government. The findings and conclusions presented in this paper are those of the authors and do not necessarily reflect the views of the NIH or the U.S. Department of Health and Human Services.

Fig. 1: Schematics of inner ear sensory epithelia and hair cells. (A) Anatomy of inner ear sensory epithelia. The cochlea detects sound stimuli, using inner and outer hair cells in the organ of Corti. The vestibule detects acceleration acting on our body, including gravity, using hair cells in the utricle, saccule, and cristae ampullares. (B) Stereocilia architecture highlighting the contribution of myosin VIIA (MYO7A) during the development of the mechanoelectrical transduction (MET) machinery. The motor activity of MYO7A is essential for transporting proteins to the upper tip-link density (UTLD), which tethers tip links connected to MET channels. Deflection toward the side of longer stereocilia opens MET channels via tip links.
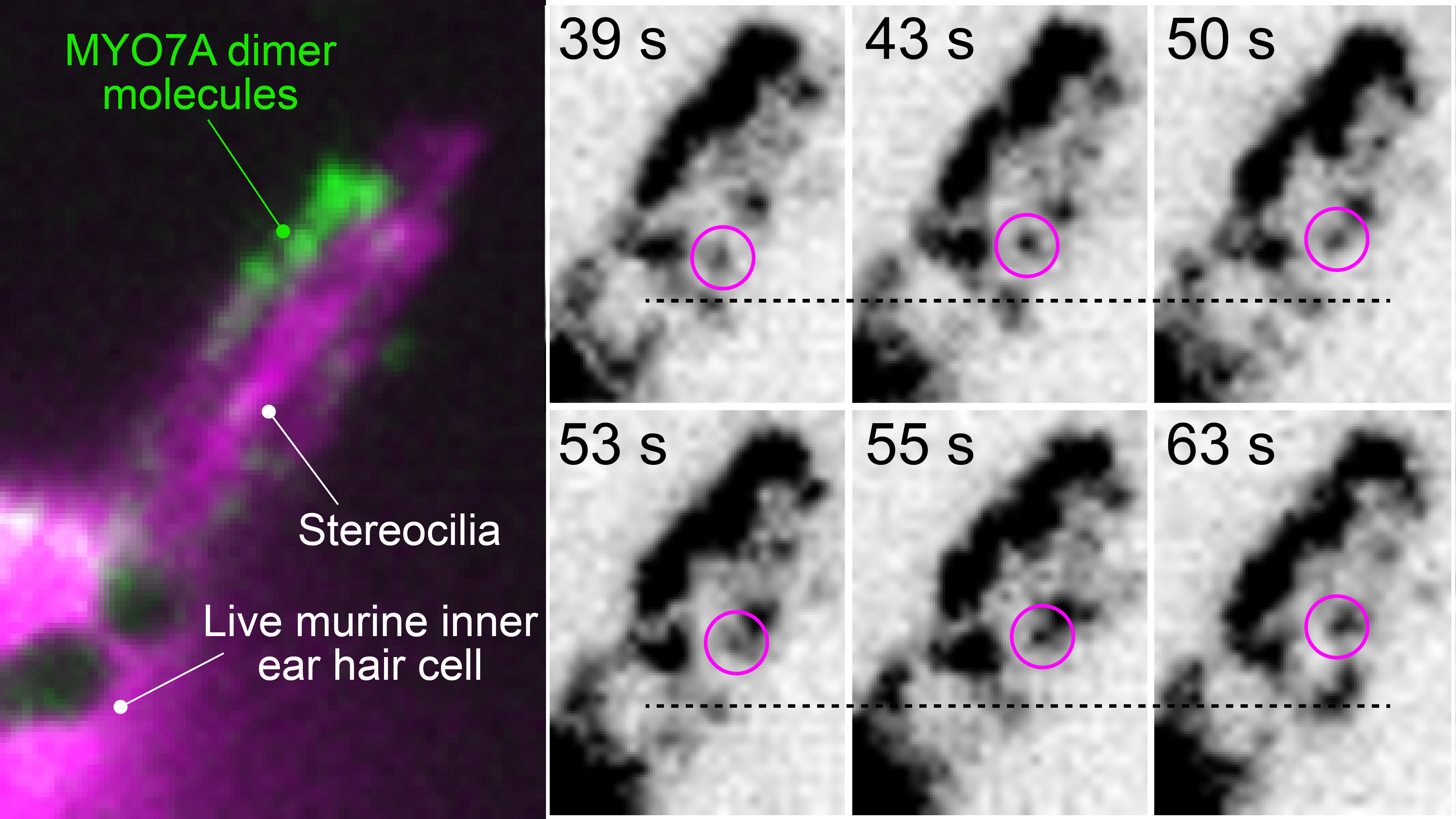
Fig. 2: Single-molecule imaging of MYO7A dimers moving within stereocilia of live murine hair cells. The entire architecture of stereocilia is visualized by co-expressed EGFP (pseudocolored in magenta). Time-lapse images highlight a molecule of MYO7A moving toward stereocilia tips. See Movie.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in