N, N’-bidentate ligand anchored palladium catalysts on MOFs for efficient Heck reaction
Published in Materials
The Heck reaction is a cornerstone method for constructing C-C bonds in organic synthesis and the pharmaceutical industry. Since Mizoroki and Heck's groundbreaking work in the 1970s, where they reported the palladium-catalyzed cross-coupling of iodobenzene and olefin, the Heck reaction has become an indispensable tool in organic chemistry. The cinnamate esters produced through this reaction are widely used as food additives and serve as crucial intermediates in the synthesis of the anticancer drug paclitaxel.
In conventional Heck reaction systems, homogeneous palladium catalyst is commonly used as a catalyst. However, challenges such as recycling difficulties, high costs, and environmental pollution are frequently cited. Additionally, the presence of residual metals in final products poses a significant obstacle to the use of transition-metal catalysts in pharmaceutical synthesis. Therefore, the development of suitable heterogeneous catalysts is essential to overcome these challenges, protect the environment, and advance the principles of green chemistry.
In recent decades, researchers have extensively studied the incorporation of metal catalysts into metal-organic frameworks (MOFs) to develop effective heterogeneous catalysts. A common strategy involves coordinating catalytic centers with 2,2'-bipyridine-based ligands to form five-membered ring structures. Recently, we discovered that the two nitrogen atoms in the bipyridine ligand are part of a large π system, creating a rigid structure that could hinder optimal overlap with palladium's electron clouds. We proposed that the flexibility of the ligand's anchoring groups is crucial for maintaining crystallinity during metalation and coordination with metal centers. Thus, we have devised and synthesized a multifunctional ligand, 3-amino-4-(2-pyridyl) benzoic acid (APBA), derived from the 2-(2-aminophenyl) pyridine ligand, as illustrated in Figure 1. Compared to 2,2'-bipyridine, APBA offers enhanced flexibility in coordinating with palladium, thanks to the rotatable C–N bond in its amino group. Pd@MOF-APBA was synthesized according to Figure 1.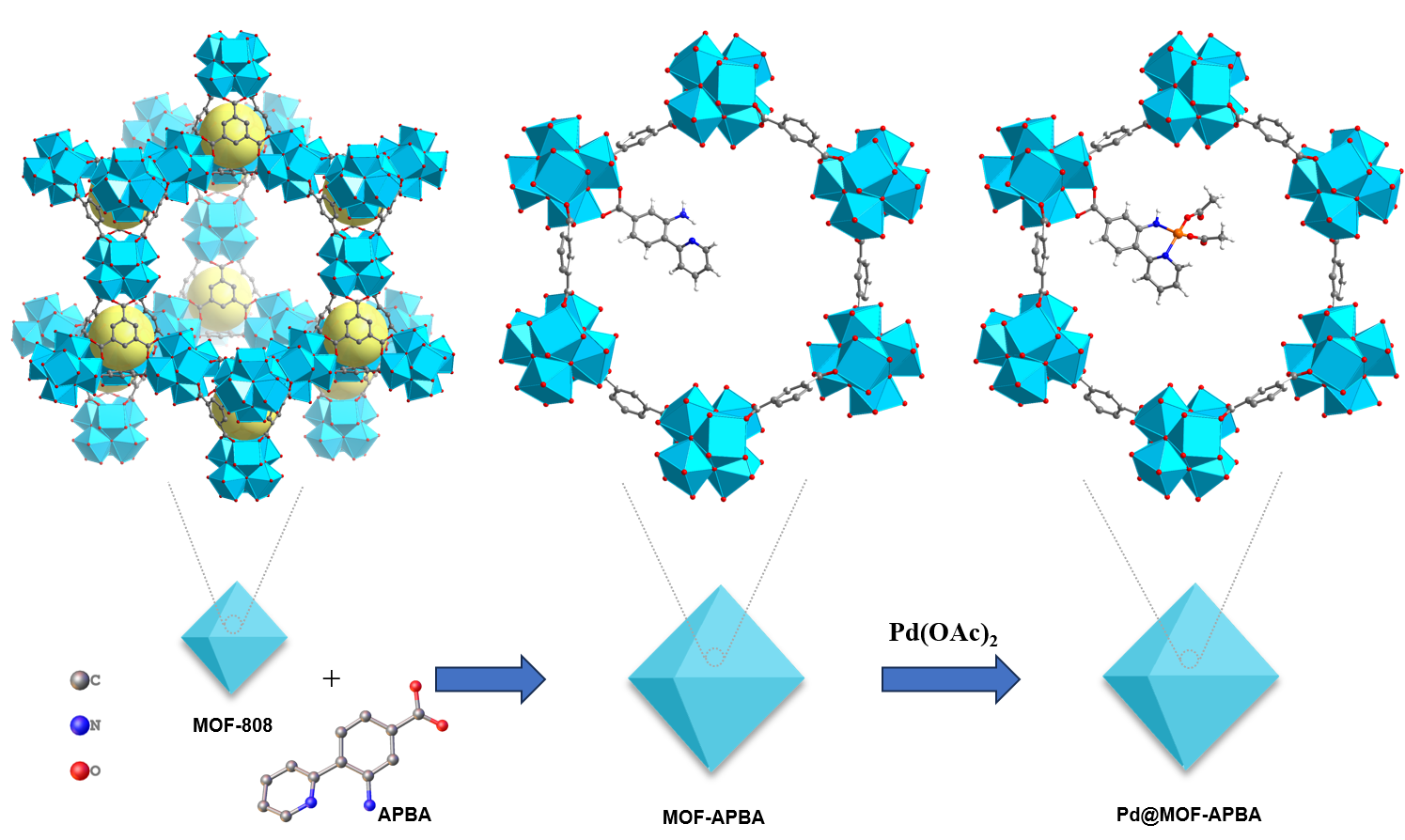
We characterized Pd@MOF-APBA using XRD, ICP, SEM, TEM, XPS, and XAS. To further elucidate its structure and catalytic activity, we also synthesized Pd@MOF-808, PdCl2@MOF-APBA, and Pd@MOF-BCA for comparison. The results demonstrated that the chelation of N, N'-bidentate ligands in Pd@MOF-APBA effectively immobilizes the palladium catalytic centers within MOF-808, postponing palladium leaching and agglomeration while maintaining high catalytic activity. Additionally, N2 adsorption-desorption isotherms confirmed that the ample pore size of MOF-808 can accommodate both APBA and palladium centers, providing sufficient space for catalytic reactions.
The synthesized catalyst exhibited excellent catalytic performance in the Heck cross-coupling reaction, achieving a high turnover number (TON) exceeding 95,000. Moreover, the catalyst can be recycled at least four times with a 100% yield, and up to three times even in gram-scale reactions, presenting itself as a promising candidate for heterogeneous catalysis in the pharmaceutical industry.
I would like to extend my gratitude to all the authors for their contributions to this work. Special thanks to Prof. Shuai Yuan, Prof. Yuanyuan Wang, the editor, and the reviewers for their invaluable suggestions throughout this project.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in