Nanovesicles loaded with a TGF-β receptor 1 inhibitor overcome immune resistance to potentiate cancer immunotherapy
Published in Healthcare & Nursing

Background
Tumors are frequently distinguished by three basic immune profiles1, among these, the immune-excluded phenotypes exhibit the presence of abundant immune cells retained in the stroma that surrounds nests of tumor cells but lack infiltrating T cells into the tumor2. Immune-excluded tumors (IETs) are resistant to many kinds of treatments, indicating the presence of multiple immune-tolerance mechanisms3.
Transforming growth factor-β (TGF-β) signaling is an important mechanism of immune suppression in the tumor microenvironment. It promotes the production of fibroblast matrix collagen by regulating the phosphorylation of Smad2/3 protein to form a “physical barrier”, which affects the infiltration of T cells into tumor tissues, leading to intrinsic immune resistance4,5. In addition, interferon-gamma (IFNγ)-mediated upregulation of immunosuppressive factors such as programmed death ligand 1 (PD-L1) and indoleamine 2,3-dioxygenase 1 (IDO1) can impair T cell function, and cause adaptive immune resistance6. Many studies have demonstrated that JQ1 reversed IFNγ-stimulated PD-L1 activation by inhibiting bromodomain-containing protein 4 (BRD4) activation7-9. However, there remain unsurmountable challenges to overcoming intrinsic and adaptive immune resistance at the same time.
How did we investigate this?
By bioinformatic analysis, we found TGFBR1 is significantly upregulated in a broad spectrum of solid tumors. And it suppresses the intratumoral infiltration levels of T lymphocytes and activated dendritic cells (DCs) while increasing the infiltration levels of the regulatory T cells (Tregs) and cancer-associated fibroblasts (CAFs). These results demonstrate that the activated TGF-β signaling pathway is one of the factors which promote the deposition of extracellular matrix, called the “physical barrier”, and hinders the intratumor infiltration of the cytotoxic T lymphocytes (CTLs). Then, we found the TGF-β receptor 1 inhibitor LY could relieve tumor fibrosis, thus recruiting tumor-infiltrating T lymphocytes unexpectedly. To realize the tumor-specific drug co-delivery, an enzyme-activated nanovesicle containing LY and photosensitizer pyropheophorbide a (PPa) was designed. However, the anti-tumor therapy demonstrated that it enhanced the number of tumor-infiltrating T cells, and inhibited tumor growth in the earliest stages of tumor development, but may not be fully eradicated, resulting in possible tumor recurrence and metastasis. Next, we inquired into possible causes for the failure of the treatment that we see. Unfortunately, the tumor-infiltrating effector T cells induced the overexpression of PD-L1 on the surface of tumor cells by secreting IFNγ, which in turn, caused inducible immune resistance. Finally, these nanovesicles are further armored with a lipophilic prodrug of the BRD4 inhibitor JQ1 in hopes of abolishing PD-L1 expression of tumor cells and conquering the intrinsic and adaptive immune resistance simultaneously.
Key findings
We found that the TGF-β receptor 1 inhibitor LY inhibited tumor fibrosis and relieved the dense “physical barrier” of the tumor tissue to promote the intratumoral infiltration of the T lymphocytes.
Furthermore, a tumor enzymatic microenvironment-activatable nanovesicle, namely ELNV, was constructed for the tumor-specific co-delivery of LY and a photosensitizer PPa. We found that the LY-loaded nanovesicles efficiently inactivated cancer-associated fibroblasts and suppressed tumor fibrosis by blocking the TGF-β signaling pathway, which promoted the intratumoral infiltration of T lymphocytes (Figure 1).
Then, ELNV was further armored with a reactive oxygen species-activatable lipophilic prodrug of the BRD4 inhibitor JQ1. The findings showed that JQ1 prodrug-integrated nanovesicles efficiently abolished the IFN-γ-inducible PD-L1 expression on the surface of the tumor cell membrane and thus conquered the adaptive immune resistance (Figure 1).
Take home message
TGF-β signaling plays an important role in tumor initiation, progression, and tumor immune evasion. It is crucial to find ways for blocking TGF-β signaling, therefore combatting immune resistance. In this study, the bidirectional regulation nanovesicles (ELJNV) were designed to combat intrinsic and inducible immune resistance. These multifunctional nanovesicles could activate T cells and relieve the inhibitory effects of the immunosuppressive tumor microenvironment (ITM) on T cells. It was found that the combination of TGF-β receptor inhibitor and JQ1 with photodynamic therapy (PDT) could stimulate the recruitment of T cells, modulate fibrosis in tumor tissues, and reduce PD-L1 expression on the surface of tumor cells, which synergistically facilitates CTLs infiltration and improves the effectiveness of photodynamic-immune combination therapy against multiple tumor models including the IETs and hot tumors (Figure 1). Nanotechnology derived from these intelligent nanovesicles might become a general solution for the targeted co-delivery of insoluble photosensitizers, small molecule immunomodulators, and inhibitors of immune negative regulators. Furthermore, the combination of antifibrotic therapies and immune-enhancing therapies would provide an effective and safe method for promoting immunotherapies, such as immune-checkpoint blockade (ICB), CAR-T, and vaccines and overcoming immune evasion against malignant tumors.
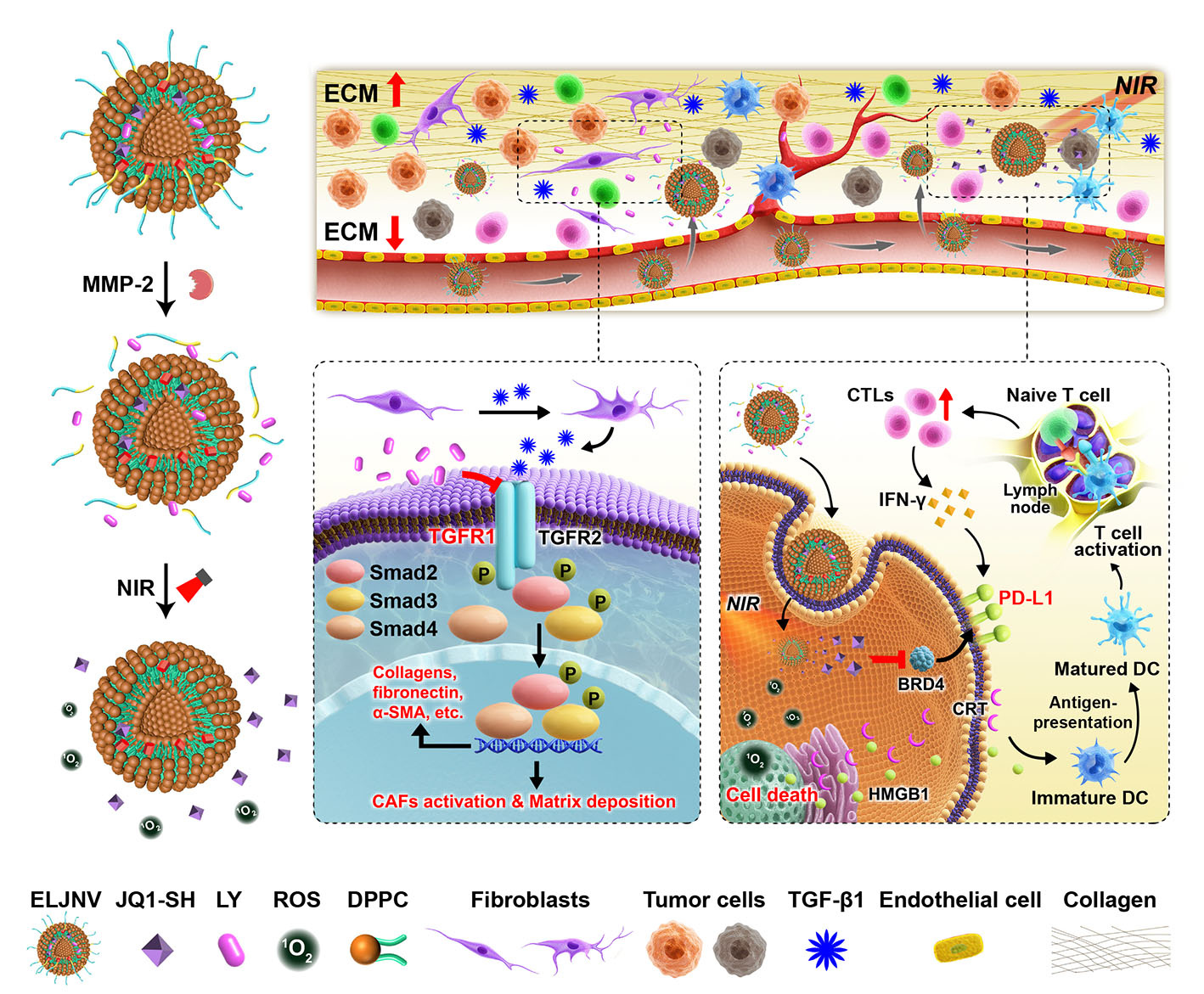
Figure 1: Schematic of the nanovesicles to avoid the immune resistance of IETs. This study reports stimuli-activatable nanovesicles (ELJNV) to circumvent cancer immune evasion by blocking the TGF-β signaling pathway and suppressing PD-L1 expression. The nanovesicles promoted intratumoral CTLs infiltration by relieving the extracellular matrix, followed by PDT-recruitment of more CTLs into the solid tumors and eventually ride over the IFNγ-induced PD-L1 expression.
For more detail on the experiments and results, please read our paper https://doi.org/10.1038/s41467-023-39035-x
References
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 140, 883-899 (2010).
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321-330 (2017).
- Chiu HW, Ho SY, Guo HR, Wang YJ. Combination treatment with arsenic trioxide and irradiation enhances autophagic effects in U118-MG cells through increased mitotic arrest and regulation of PI3K/Akt and ERK1/2 signaling pathways. Autophagy 5, 472-483 (2009).
- Meng X-m, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat. Rev. Nephrol. 12, 325-338 (2016).
- Pinter M, Jain RK. Targeting the renin-angiotensin system to improve cancer treatment: Implications for immunotherapy. Sci. Transl. Med. 9, eaan5616 (2017).
- Patel SA, Minn AJ. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 48, 417-433 (2018).
- Zhou F, et al. Engineering Chameleon Prodrug Nanovesicles to Increase Antigen Presentation and Inhibit PD-L1 Expression for Circumventing Immune Resistance of Cancer. Adv. Mater. 33, e2102668 (2021).
- Zhou F, et al. Overcoming immune resistance by sequential prodrug nanovesicles for promoting chemoimmunotherapy of cancer. Nano Today 36, 101025 (2021).
- Ye J, et al. Bispecific prodrug nanoparticles circumventing multiple immune resistance mechanisms for promoting cancer immunotherapy. Acta Pharm. Sin. B 12, 2695-2709 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in