Neural mechanisms of emotional health in traumatic brain injury patients undergoing rTMS treatment
Published in Neuroscience

My interest in an injured brain
My interest in Computational Neuroscience began in 2018 when I started as a PhD student at NED University of Engineering and Technology, located in Karachi, Pakistan. I was the only female engineer at NED University who focused on brain imaging for a PhD thesis. Brain injury is common in Pakistan, especially in a large metropolitan city like Karachi where motor vehicle accidents are the main cause of traumatic brain injury (TBI). Although diagnosis and treatment for brain injury is available in hospitals around the city, I was more intrigued by how the brain changes after such an injury. Through my mentors, Dr. Saad Ahmed Qazi and Dr. Abul Hasan at NED University, I was introduced to Dr. Adeel Razi at Monash University, Australia and Dr. Maheen Adamson at VA Palo Alto (VAPA) and Stanford University, USA, both working in neuroimaging and brain injury. My first review paper with them reported the incidence of brain injury in Pakistan and its association with dementia (1). At this point, my engineering background led me towards understanding the intricacies of the injured brain and the changes in quality of life after injury.
A great collaboration for a noble cause
After this review publication and the annual Organization of Human Brain Mapping Conference (2020), I became part of a collaboration between Drs. Adamson and Razi. Specifically, neuroimaging data from United States (US) Veterans with TBI was available for further analysis before and after neuromodulation treatment and Dr. Razi had developed a new method for inferring changes in brain connectivity from MRI scans. In the dataset, repetitive Transcranial Magnetic Stimulation (rTMS), clinically utilized for Major Depressive Disorder, was used to improve executive function and quality of life in these Veterans with TBI at VAPA. My interests, however, involved not just TBI but also the emotional impact it has on the lives of these Veterans. TBI is a signature injury of the warzone, and nearly a half million service members and Veterans in the US have been diagnosed with TBI. Emotion dysregulation is among the most common long-term consequence of traumatic brain injury (TBI) that have a strong impact on the quality of life of the patient (2). rTMS is emerging as a treatment for post-concussive symptoms specifically depression, however, previous research was mostly focused on psychiatric disorders (3-8).
So what sort of dataset did we use?
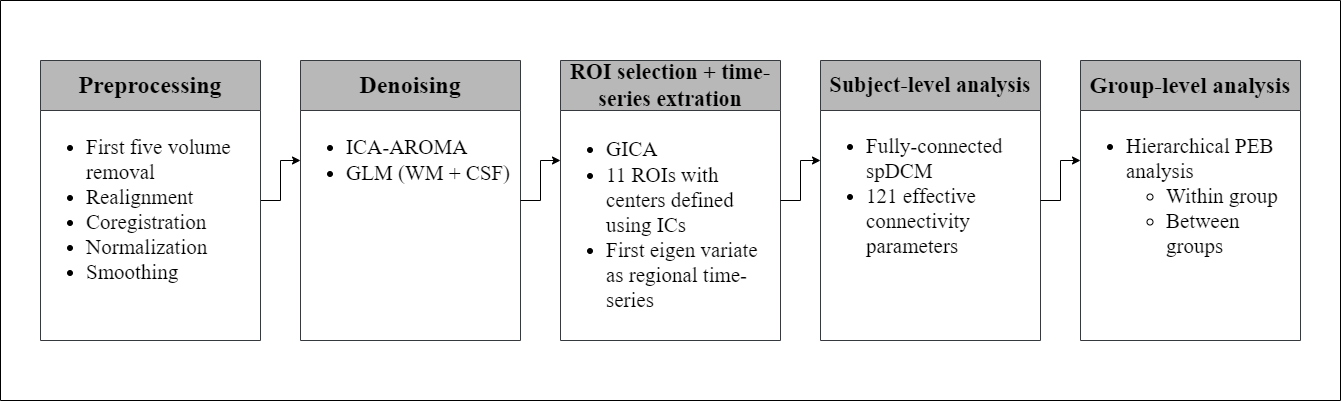
In collaboration with Neurocomputation Lab (NCAI) (NED University), VAPA and Monash University, Australia, I inferred the changes in brain effective connectivity and its association with emotional health improvement in TBI after an application of rTMS. The dataset comprised of resting-state fMRI scans of 32 veterans with chronic mild/moderate TBI and their emotional health scores. It was a double-blind randomized controlled clinical trial; hence, the subjects were divided into active and sham group. Treatment was administered over left DLPFC with intensity of 120% of motor threshold in 80 5-second trains at 10 Hz frequency with 10-second inter-train interval (20 sessions in total). The intensity of the rTMS stimulation was determined by using TMS motor threshold assessment tool. The entire acute treatment phase (20 sessions) normally took 2 weeks with 2-3 treatments each day. MRI scans were conducted at baseline and post-treatment. MRI scans and other self-report questionnaires were also completed within 2-5 hours of the end of treatment.
What analysis tools (algorithms/models) were applied?
Our team hypothesized that treatment with rTMS would yield improved emotional health in TBI subjects, and based on the previous functional connectivity literature, the effective connectivity between the selected tri-network would play major a role in the emotional wellness. Dynamic causal modelling (DCM) (9-10) is the preferred approach for the analysis of effective connectivity using multivariate neural time series from various brain regions of interest. We used its variant called spectral DCM (11-12) for subject-level analysis, which is widely adopted to model the directed communication among brain regions in the `resting-state’. Parametric empirical Bayes (PEB) – a hierarchical Bayesian inference method (13) – was used to perform the group-level analysis; to observe the differences in effective connectivity pre and post treatment, in the active and sham group separately, and to find the association between emotional health scores and the effective connectivity.
What did we find out?
The psychometric results yielded a significant difference between the emotional health scores of active groups pre- and post-rTMS while it was not significant in the sham group. The effective connectivity strengths in active group post-rTMS, were decreased for excitatory connections and increased for inhibitory connections among inter-areal connections. The connectivity differences between pre- and post-rTMS were also found in sham group. Interestingly, the strength of all the excitatory and inhibitory connections decreased and increased respectively as it did in the active group. These connectivity patterns perhaps indicate the placebo effect where the overall excitatory influence decreased while the overall inhibitory influence increased between the regions after sham treatment. The association analysis of pre-rTMS connectivity with emotional health scores revealed 21 connections before the treatment while there were only 2 connections after the treatment (active group only) that are associated with the emotional health scores. In the active group, we found dorsal anterior cingulate cortex (dACC) to medial prefrontal cortex to be positively associated at the baseline while negatively associated at post-rTMS, and dACC to left anterior insula was positively associated with the emotional health scores as shown in the figure below. Same analysis performed for post-rTMS sham group yielded no association of emotional health scores with any connection.
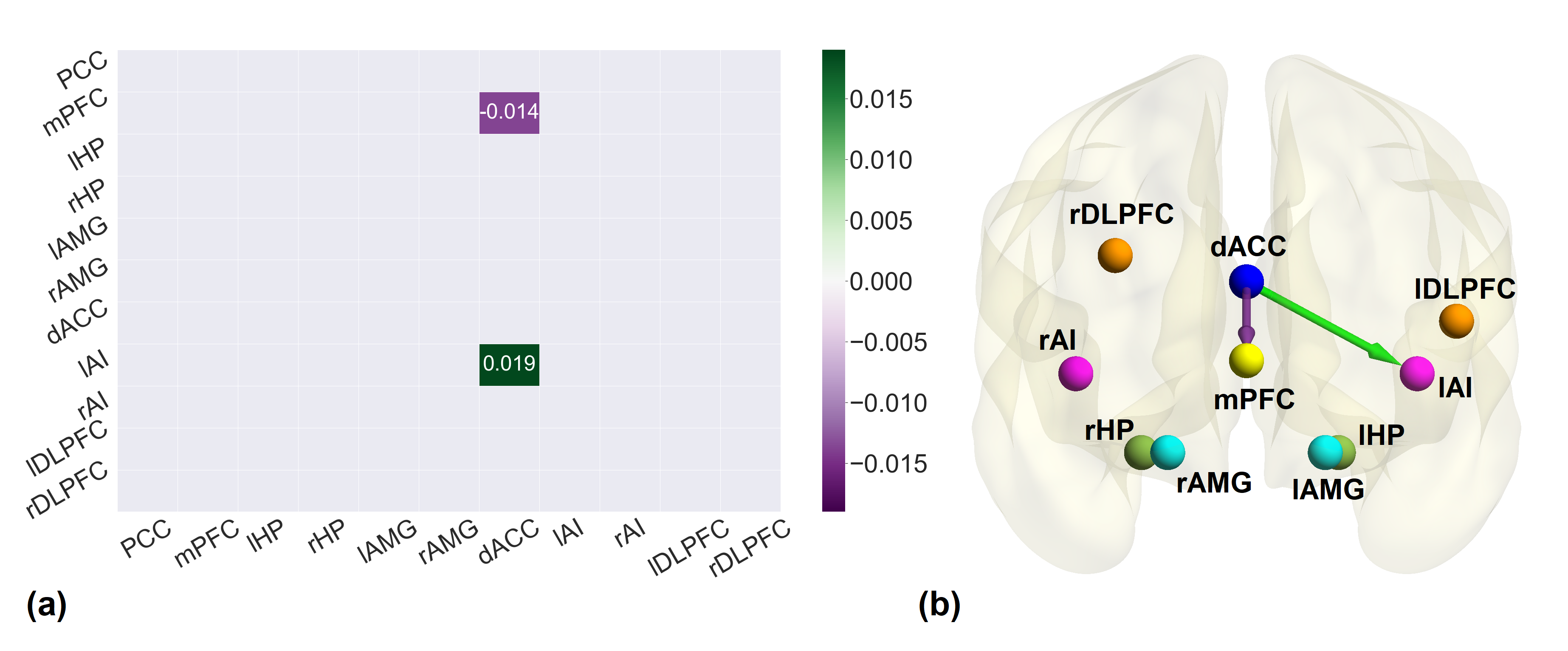
a The association matrix between post-rTMS connectivity of active group and emotional health scores. The purple gradient indicates negative association while green gradient shows positive association. b The brain diagram of the association between post-rTMS effective connectivity of active group and emotional health. Green and Purple arrows illustrate the positive and negative association respectively. All the associations reported here survived the threshold of posterior probability >0.95 amounting to a strong evidence.
Take-home message
The cardinal region in the analysis was dorsal anterior cingulate cortex which is considered to be the most influenced during emotional health disorders. Our key findings implicate the altered connectivity of dACC with left anterior insula and medial prefrontal cortex, after the application of rTMS, as a potential neural mechanism underlying improvement of emotional health. Our investigation highlights the importance of these brain regions as treatment targets in emotional processing in TBI, a devastating injury that leads to decreased quality of life.
References:
- Adamson, M. M., Shakil, S., Sultana, T., Hasan, M. A., Mubarak, F., Enam, S. A., Parvaz, M. A., & Razi, A. (2020). Brain Injury and Dementia in Pakistan: Current Perspectives. Frontiers in Neurology, 11. https://doi.org/10.3389/fneur.2020.00299
- R. Howlett, L. D. Nelson, and M. B. Stein, “Mental Health Consequences of Traumatic Brain Injury,” Biol. Psychiatry, vol. 91, no. 5, pp. 413–420, Mar. 2022, doi: 10.1016/J.BIOPSYCH.2021.09.024.
- H. Siddiqi et al., “Repetitive Transcranial Magnetic Stimulation with Resting-State Network Targeting for Treatment-Resistant Depression in Traumatic Brain Injury: A Randomized, Controlled, Double-Blinded Pilot Study,” J. Neurotrauma, vol. 36, no. 8, pp. 1361–1374, Apr. 2019, doi: 10.1089/neu.2018.5889.
- Stilling et al., “Treatment of Persistent Post-Traumatic Headache and Post-Concussion Symptoms Using Repetitive Transcranial Magnetic Stimulation: A Pilot, Double-Blind, Randomized Controlled Trial,” J. Neurotrauma, vol. 37, no. 2, pp. 312–323, Jan. 2020, doi: 10.1089/NEU.2019.6692.
- E. Hoy, S. Mcqueen, D. Elliot, S. E. Herring, J. J. Maller, and P. B. Fitzgerald, “A Pilot Investigation of Repetitive Transcranial Magnetic Stimulation for Post-Traumatic Brain Injury Depression: Safety, Tolerability, and Efficacy,” J. Neurotrauma, vol. 36, no. 13, pp. 2092–2098, Jul. 2019, doi: 10.1089/neu.2018.6097.
- Rao et al., “Low-frequency right repetitive transcranial magnetic stimulation for the treatment of depression after traumatic brain injury: A randomized sham-controlled pilot study,” J. Neuropsychiatry Clin. Neurosci., vol. 31, no. 4, pp. 306–318, Oct. 2019, doi: 10.1176/appi.neuropsych.17110338.
- H. Siddiqi et al., “Individualized connectome-targeted transcranial magnetic stimulation for neuropsychiatric sequelae of repetitive traumatic brain injury in a retired NFL player,” J. Neuropsychiatry Clin. Neurosci., vol. 31, no. 3, pp. 254–263, Jul. 2019, doi: 10.1176/appi.neuropsych.18100230.
- A. Lee and M. K. Kim, “Effect of Low Frequency Repetitive Transcranial Magnetic Stimulation on Depression and Cognition of Patients with Traumatic Brain Injury: A Randomized Controlled Trial,” Med. Sci. Monit., vol. 24, p. 8789, Dec. 2018, doi: 10.12659/MSM.911385.
- Razi and K. J. Friston, “The Connected Brain: Causality, models, and intrinsic dynamics,” IEEE Signal Process. Mag., vol. 33, no. 3, pp. 14–55, May 2016, doi: 10.1109/MSP.2015.2482121.
- J. Friston, L. Harrison, and W. Penny, “Dynamical Causal Modelling,” Neuroimage, vol. 19, no. 4, pp. 1273–1302, 2003, doi: 10.1016/S1053-8119(03)00202-7.
- J. Friston, J. Kahan, B. Biswal, and A. Razi, “A DCM for resting state fMRI,” Neuroimage, vol. 94, pp. 396–407, Jul. 2014, doi: 10.1016/j.neuroimage.2013.12.009.
- Razi, J. Kahan, G. Rees, and K. J. Friston, “Construct validation of a DCM for resting state fMRI,” Neuroimage, vol. 106, pp. 1–14, Feb. 2015, doi: 10.1016/j.neuroimage.2014.11.027.
- Zeidman, P., Jafarian, A., Seghier, M. L., Litvak, V., Cagnan, H., Price, C. J., & Friston, K. J. (2019). A guide to group effective connectivity analysis, part 2: Second level analysis with PEB. NeuroImage, 200, 12–25. https://doi.org/10.1016/j.neuroimage.2019.06.032
Follow the Topic
-
Molecular Psychiatry

This journal publishes work aimed at elucidating biological mechanisms underlying psychiatric disorders and their treatment, with emphasis on studies at the interface of pre-clinical and clinical research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcement


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in