New Battery Interphase Technology: Electrophile Reduction Strategy
Electric energy has become the foundation of modern life and technologies, from smart devices to electric transportation and AI. Batteries are central to meeting growing energy demands and advancing sustainable energy. However, reports of EV fires and battery plant explosions have raised significant concerns, hindering the widespread adoption of batteries. Beyond safety issues, current battery technologies are also limited by their life and energy densities.
All-solid-state lithium metal batteries (ASSLMBs) are considered next-generation energy storage technologies, offering superior energy density and enhanced safety compared to conventional lithium-ion batteries. This advantage arises from the use of inorganic solid-state electrolytes (SSEs), which are mechanically robust and non-flammable. However, no single SSE has yet met all the properties required for practical application (Fig. 1). Oxide SSE has a wide electrochemical stability window, but their high mechanical strength leads to poor contact with electrodes and challenges in large-scale manufacturing. Halide SSE have good electrode contact and excellent high-voltage stability but are extremely unstable on the anode side, limiting their application to catholytes. Among them, sulfide SSE is regarded as the most promising for commercialization, possesses very high ionic conductivity, good electrode contact, and ease of manufacturing. However, they have a narrow electrochemical stability window, showing low compatibility with both high-voltage cathodes and lithium anodes. Meanwhile, there is a critical challenge across all SSEs, lithium dendrite, needle-like structures that can penetrate the electrolyte, leading to cell short circuits and failure. Current approaches to mitigate dendrites rely on high pressure and/or pressures, which are impractical for industrial applications.
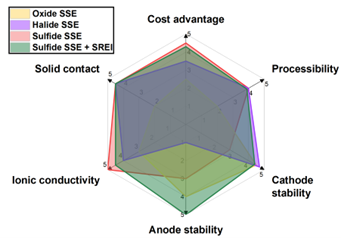
Limitations of Current Strategies
Extensive research has been dedicated to solid-state batteries, with numerous companies racing to become the first to commercialize them. Unlike liquid electrolytes, which can be directly modified by adjusting solvents, salts, additives, or solvation structures, inorganic SSEs have limited compositional flexibility, making it challenging to directly enhance their electrochemical stability. As a result, most existing research has focused on modifying the structure of batteries by introducing artificial interlayers between solid electrolytes and electrodes. While effective, these interlayers increase costs, reduce processability, and degrade electrode-electrolyte contact. Additionally, high-temperature and/or high-pressure conditions remain necessary.
A New Strategy: Electrophile Reduction
Therefore, a strategy that can directly modify the chemical and electrochemical properties of solid electrolytes is highly desirable. Such a strategy would enable direct resolution of these issues and allow rapid adoption in commercial applications without altering existing battery structures. In this work, we propose the “electrophile reduction” strategy. This concept is based on a family of reductive electrophiles that gain electrons and cations from solid electrolytes upon contact, becoming electrochemically reduced to form a dense, electron-blocking layer on material surfaces (Fig. 2).
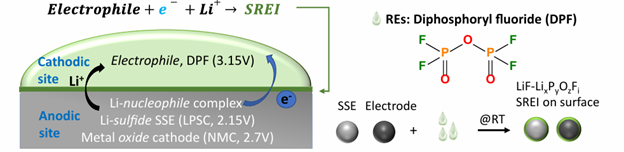
The Story Behind the Electrophile Reduction Concept
Although this work focuses on all-solid-state batteries, the underlying concept stems from our understanding of electrochemistry in liquid electrolytes. In liquid batteries, the electrolyte is commonly reduced by gaining electrons from the anode at low potential, forming a solid electrolyte interphase (SEI) to prevent further electrolyte decomposition and stabilize the electrode. Previous studies highlighted that reducing the organic content in SEI and increasing the LiF concentration could weaken interactions with the lithium metal anode and enhance stability. Inspired by this, we sought to design a fluorine-rich, fully inorganic electrolyte solvent, which led to the synthesis of diphosphoryl fluoride, the core component of this work. We discovered that diphosphoryl fluoride acts as a reductive electrophile with strong electron affinity and high reduction potential, while its high reactivity makes it unsuitable for the main solvent for lithium metal batteries. However, these properties offer significant advantages for stabilizing solid electrolytes.
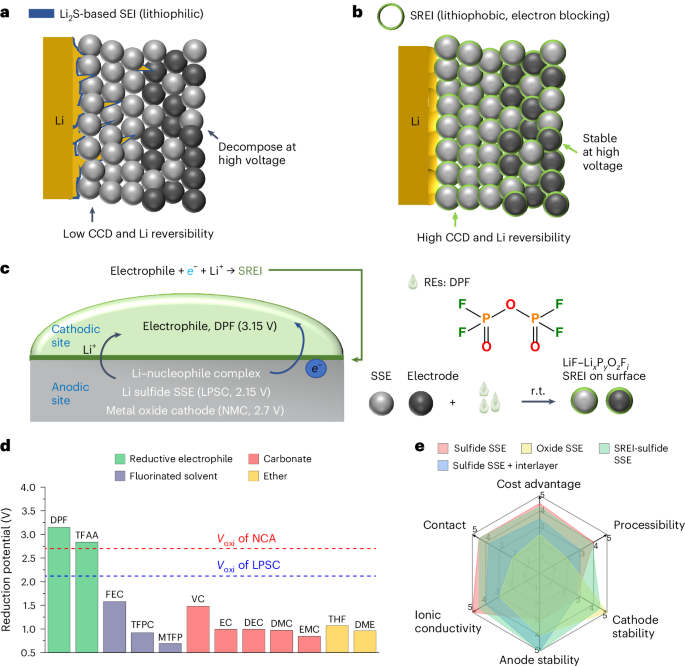
Solid-state electrolytes have limited composition flexibility, making surface coating the most direct way to enhance their electrochemical stability. However, coating SSEs presents significant challenges. Conventional chemical coatings often produce non-uniform, thick layers that hinder lithium-ion conduction. In contrast, the history of lithium-ion batteries shows that SEIs in liquid batteries are typically thin, uniform, and provide low interfacial resistance, making them highly effective. Unfortunately, replicating SEI-like layers on SSE surfaces is fundamentally difficult. SEIs are generally formed on low-potential electrodes, such as graphite or silicon anodes, during the discharge process when the electrode potential drops below the reduction potential of the liquid electrolyte. For SSEs, replicating this process would require mixing the electrolyte with conductive carbon to create an electrode, assembling a cell with liquid electrolyte, and discharging the SSE to a lower potential than liquid electrolytes. These steps are impractical for constructing SEI-like layers on SSEs.
Diphosphoryl fluoride overcomes this limitation due to its high reduction potential (3.15 V), which is significantly higher than the potential of SSEs (~2.15 V), and its strong electron-attracting ability. When in contact with SSEs, diphosphoryl fluoride spontaneously gains electrons and is reduced on the electrolyte surface, forming a stable, dense layer akin to SEI. This layer greatly enhances the chemical and electrochemical stability of SSEs.
This strategy extends beyond SSEs. We also demonstrated that reductive electrophiles could form similar protective layers on oxide-based cathode materials, as their reduction potential is even higher than cathode materials (~2.7V). This enhances the high-voltage stability of cathode materials. Building on this principle, we identified a family of reductive electrophile molecules. Given its broad applicability, surpassing the traditional SEI, we named this interphase, formed by reductive electrophiles, the solid reductive electrophile interphase (SREI).
Impact and practical implication
The SREI greatly improves the electrochemical stability of SSEs, significantly suppresses lithium dendrite growth, and enables high-performance ASSLMBs to operate at room temperature and low pressure. This breakthrough addresses longstanding doubts about the practicality of ASSLMBs, marking a significant advancement in their development.
Moreover, the treatment process for SREI is straightforward and does not require advanced equipment. Since it directly modifies the electrolyte, it does not alter existing battery manufacturing processes, offering immense potential for industrial scalability.
Beyond Batteries
The implications of this work extend beyond batteries. By leveraging the versatility of reductive electrophiles, we can develop inorganic materials with targeted chemical and electrochemical properties, opening new possibilities for inorganic materials with limited composition formulas. This strategy represents a seamless integration of organic chemistry with inorganic materials, providing innovative solutions for energy storage and materials engineering.
More details of this study can be found in our recent article "Revitalizing interphase in all-solid-state Li metal batteries by electrophile reduction" published in Nature Materials.
Zhang, W., Wang, Z., Wan, H. et al. Revitalizing interphase in all-solid-state Li metal batteries by electrophile reduction. Nat. Mater. (2025).
DOI: https://doi.org/10.1038/s41563-024-02064-y
Contributors: Weiran Zhang & Chunsheng Wang
Follow the Topic
-
Nature Materials

A monthly multi-disciplinary journal that brings together cutting-edge research across the entire spectrum of materials science and engineering, including applied and fundamental aspects of the synthesis/processing, structure/composition, properties and performance of materials.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in