pH-Responsive Upconversion Mesoporous Silica Nanospheres for Enhanced Phototherapy
Published in Cancer
Explore the Research
acsnano.3c04564?utm_campaign=related_content&utm_source=HEALTH&utm_medium=communities
Conventional cancer treatments, such as chemotherapy, radiotherapy, and surgery suffer from a number of limitations, including a range of side-effects and complications, immunosuppression, development of multidrug resistance (MDR) phenotypes, recurrence, and metastasis [1-3]. As a result, there has been considerable attention directed towards developing alternative, effective therapeutic approaches to supplement or replace existing cancer treatments. Foremost among these alternatives are light-based therapies, photodynamic therapy (PDT) and photothermal therapy (PTT), owing to their non-invasive nature and high efficiency [4, 5]. PDT uses laser irradiation to activate a photosensitizer (PS) that generates cytotoxic reactive oxygen species (ROS), through a series of photochemical reactions, to induce apoptosis in cancer cells, while in PTT a photothermal agent (PTA) is used to convert absorbed light into heat and the resulting hyperthermia leads to the partial or complete ablation of tumor tissue [5, 6].
Many of the common PS and PTA molecules however, are characterized by poor solubility, rapid in vivo degradation and clearance, and lack of tumor specificity, thereby hindering the clinical application of PDT and PTT [5, 7] These characteristics are problematic given that ROS is a highly reactive molecule with a very short lifetime and a limited radius of action, making it necessary for sufficient amounts of PS molecules to be present in the tumor tissue for effective PDT [7, 8]. Likewise, the localized hyperthermia required for PTT is dependent on significant accumulation of PTAs within tumors [6, 7]. It has also been reported that using either PDT or PTT alone is insufficient to completely ablate tumors and often leads to the recurrence of tumors; since most PS molecules require the use of oxygen to generate toxic ROS, the often hypoxic microenvironment of tumors can impair PDT [9-11], while hyperthermia-induced overexpression of heat shock proteins, as part of the stress response, confers a degree of thermotolerance to cancer cells that ultimately attenuates the effects of PTT [12-14].
The limitations associated with PDT and PTT have prompted the development of a wide range of nanocarriers for the more effective delivery of PS and PTA molecules to tumors and as a means to carry out simultaneous PDT and PTT [5, 15]. Combining both forms of phototherapy has been reported to synergistically improve their antitumor effects as PTT-induced hyperthermia can increase the intracellular accumulation of PS molecules and oxygen concentration in the target area by improving local blood flow, which enhances the effectiveness of PDT, while PDT-generated ROS can inactivate heat shock proteins, thereby decreasing the thermotolerance of cancer cells and increasing their susceptibility to PTT [5, 15].
Yet, to date, very few of these nanocarriers for PS and PTA delivery have reached the clinical trial stage as less than 1% of passively targeted nanoparticles accumulate in tumors [16, 17]. This is due, in large part, to the formation of a serum protein corona on the surface of nanocarriers while in circulation [18], which destabilizes nanocarriers and triggers an immune response that leads to rapid blood clearance [19, 20]. Finally, for the small fraction of nanocarriers that reach the target tumor tissue, uptake into cancer cells represents a major challenge as nanoparticles can become entrapped in endocytic or lysosomal compartments, thereby causing the loaded cargo to either undergo exocytosis via recycling endosomes, or degradation in lysosomes [17, 21].
In light of this, there is a growing body of research focused on pH-sensitive peptides that target the acidic microenvironments of tumors, promote deep tumor penetration and escape from acidic endocytic components [22, 23]. In one of our previously published studies [24], we reported increased tumor targeting and cellular uptake of nanoparticles functionalized with the acidity-triggered rational membrane (ATRAM) peptide, a second-generation pH-responsive peptide developed by our collaborators, the Barrera lab at the University of Tennessee at Knoxville. This cancer targeting peptide (CTP) was characterized as less aggregation prone compared to pH (low) insertion peptides (pHLIPs) and demonstrated effective cancer cell targeting under the mildly acidic conditions of most tumor microenvironments [25]. Therefore, combining nanoparticles with CTPs can potentially lead to more selective and efficient delivery systems for cancer therapy, resulting in improved clinical outcomes.
Inspired by stimuli-responsive nanocarriers and the ability of ATRAM to enhance the targeting and internalization of coupled nanoparticles, we wanted to resolve the issues associated with PDT and PTT by designing biocompatible and biodegradable tumor-targeted upconversion nanospheres with imaging capabilities. As reported in our paper, pH-Responsive Upconversion Mesoporous Silica Nanospheres for Combined Multimodal Diagnostic Imaging and Targeted Photodynamic and Photothermal Cancer Therapy, published in the ACS nano, the nanospheres consist of a sodium yttrium fluoride core doped with lanthanides (ytterbium, erbium, and gadolinium) and the PTA bismuth selenide (NaYF4:Yb/Er/Gd,Bi2Se3) within a mesoporous silica shell that encapsulates a PS, Chlorin e6 (Ce6), in its pores. NaYF4:Yb/Er converts deeply penetrating near-infrared (NIR) light to visible light, which excites the Ce6 to generate ROS, while Bi2Se3 efficiently converts absorbed NIR light to heat. Additionally, Gd enables magnetic resonance imaging (MRI). The mesoporous silica shell is also coated with lipid/polyethylene glycol (DPPC/cholesterol/DSPE-PEG) to ensure retention of Ce6 and minimize interactions with serum proteins and macrophages that hinder tumor targeting. Finally, the coat is functionalized with the ATRAM peptide to promote specific and efficient internalization into cancer cells within the mildly acidic tumor microenvironment.
Based on our findings, the multifunctional core-shell nanospheres our team has developed helped to overcome issues that have limited the efficacy of light-based therapies as they not only prevented the targeting of healthy tissues, thereby reducing harmful side effects, but also enhanced the potency of the encapsulated PTA and PS molecules by accumulating them at the desired site of action. This, in turn, enabled for effective and synergistic NIR laser light-induced PDT and PTT, which resulted in remarkable tumor shrinkage and substantially prolonged survival.
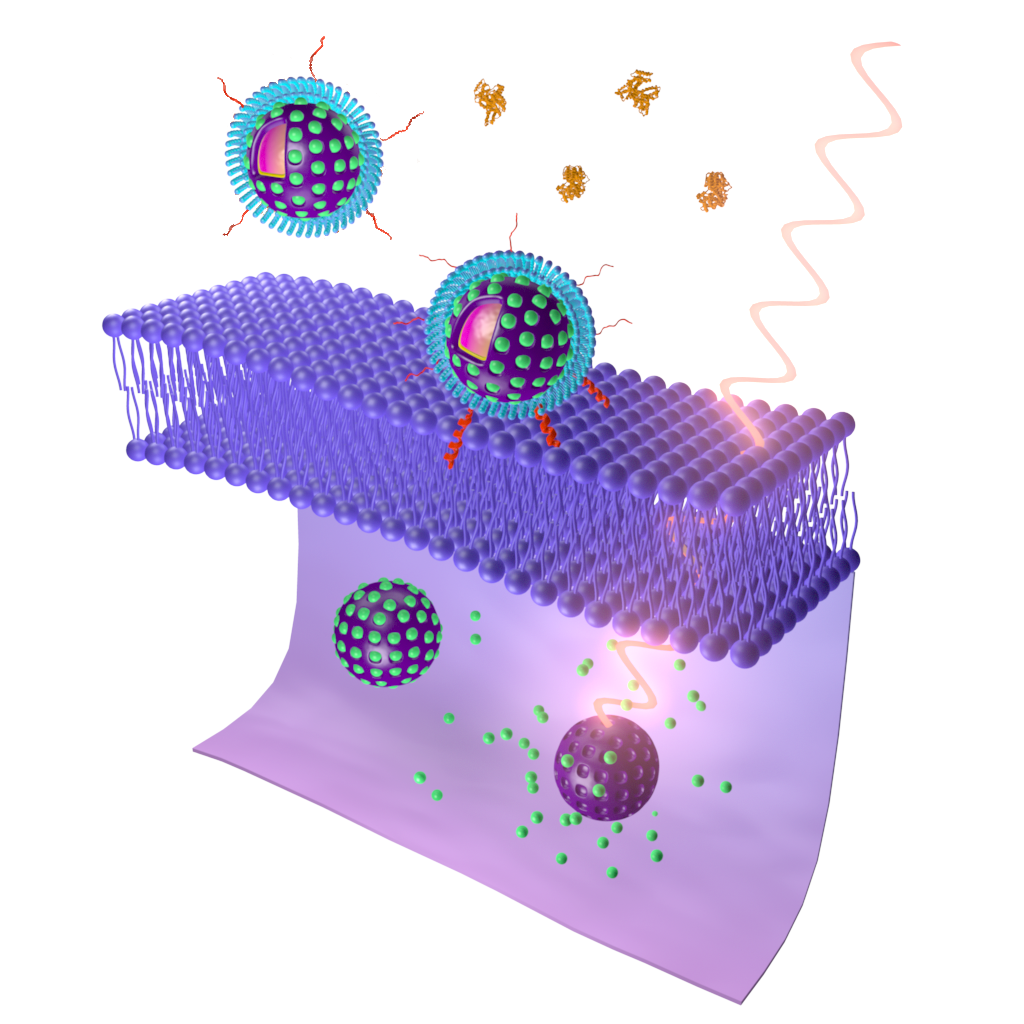
Figure 1. Schematic representation of cellular uptake of the nanospheres functionalized with ATRAM, followed by NIR laser light-induced PDT and PTT [26].
As a result, our study offers a promising tumor-targeting nanoplatform that facilitates multimodal diagnostic imaging and potent combinatorial cancer therapy. We believe that this work paves an exciting way forward for the advancement of light-based cancer treatments.
Written by Mona Kalmouni, Palanikumar Loganathan & Mazin Magzoub.
References
- Chabner, B. A., & Roberts Jr, T. G. (2005). Chemotherapy and the war on cancer. Nature Reviews Cancer, 5(1), 65-72.
- DeVita Jr, V. T., & Chu, E. (2008). A history of cancer chemotherapy. Cancer research, 68(21), 8643-8653.
- Gottesman, M. M., Fojo, T., & Bates, S. E. (2002). Multidrug resistance in cancer: role of ATP–dependent transporters. Nature reviews cancer, 2(1), 48-58.
- Agostinis, P., Berg, K., Cengel, K. A., Foster, T. H., Girotti, A. W., Gollnick, S. O., Hahn, S.M., Hamblin, M.R., Juzeniene, A., Kessel, D. and Korbelik, M. (2011). Photodynamic therapy of cancer: an update. CA: a cancer journal for clinicians, 61(4), 250-281.
- Li, X., Lovell, J. F., Yoon, J., & Chen, X. (2020). Clinical development and potential of photothermal and photodynamic therapies for cancer. Nature reviews Clinical oncology, 17(11), 657-674.
- Zhao, L., Zhang, X., Wang, X., Guan, X., Zhang, W., & Ma, J. (2021). Recent advances in selective photothermal therapy of tumor. Journal of nanobiotechnology, 19(1), 1-15.
- Deng, X., Shao, Z., & Zhao, Y. (2021). Solutions to the drawbacks of photothermal and photodynamic cancer therapy. Advanced Science, 8(3), 2002504.
- Kolarikova, M.; Hosikova, B.; Dilenko, H.; Barton-Tomankova, K.; Valkova, L.; Bajgar, R.; Malina, L.; Kolarova, H.Photodynamic Therapy: Innovative Approaches for Antibacterial and Anticancer Treatments. Res. Rev. 2023, 43 (4), 717– 774, DOI: 10.1002/med.21935
- Harris, A. L. (2002). Hypoxia—a key regulatory factor in tumour growth. Nature reviews cancer, 2(1), 38-47.
- Zhou, Z., Song, J., Nie, L., & Chen, X. (2016). Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chemical society reviews, 45(23), 6597-6626.
- Meng, Z., Xue, H., Wang, T., Chen, B., Dong, X., Yang, L., Dai, J., Lou, X. and Xia, F. (2022). Aggregation-induced emission photosensitizer-based photodynamic therapy in cancer: from chemical to clinical. Journal of Nanobiotechnology, 20(1), 1-35.
- Wang, Z., Li, S., Zhang, M., Ma, Y., Liu, Y., Gao, W., Zhang, J. and Gu, Y. (2017). Laser‐triggered small interfering RNA releasing gold nanoshells against heat shock protein for sensitized photothermal therapy. Advanced science, 4(2), 1600327.
- Wang, S., Tian, Y., Tian, W., Sun, J., Zhao, S., Liu, Y., Wang, C., Tang, Y., Ma, X., Teng, Z. and Lu, G. (2016). Selectively sensitizing malignant cells to photothermal therapy using a CD44-targeting heat shock protein 72 depletion nanosystem. ACS nano, 10(9), 8578-8590.
- Jin, Y., Liang, L., Sun, X., Yu, G., Chen, S., Shi, S., Liu, H., Li, Z., Ge, K., Liu, D. and Yang, X. (2018). Deoxyribozyme-nanosponges for improved photothermal therapy by overcoming thermoresistance. NPG Asia Materials, 10(5), 373-384.
- Overchuk, M., Weersink, R. A., Wilson, B. C., & Zheng, G. (2023). Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS nano, 17(9), 7979-8003.
- Dai, Q., Wilhelm, S., Ding, D., Syed, A. M., Sindhwani, S., Zhang, Y., Chen, Y.Y., MacMillan, P. and Chan, W.C. (2018). Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS nano, 12(8), 8423-8435.
- Kalmouni, M., Al-Hosani, S., & Magzoub, M. (2019). Cancer targeting peptides. Cellular and Molecular Life Sciences, 76(11), 2171-2183.
- Barui, A. K., Oh, J. Y., Jana, B., Kim, C., & Ryu, J. H. (2020). Cancer‐targeted nanomedicine: overcoming the barrier of the protein corona. Advanced therapeutics, 3(1), 1900124.
- Nel, A. E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E. M. V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M.Understanding Biophysicochemical Interactions at the Nano-Bio Interface. Mater. 2009, 8 (7), 543– 557, DOI: 10.1038/nmat2442
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M.Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Bioeng. Biotechnol. 2020, 8, 166, DOI: 10.3389/fbioe.2020.00166
- Patel, S., Kim, J., Herrera, M., Mukherjee, A., Kabanov, A. V., & Sahay, G. (2019). Brief update on endocytosis of nanomedicines. Advanced drug delivery reviews, 144, 90-111.
- An, M., Wijesinghe, D., Andreev, O. A., Reshetnyak, Y. K., & Engelman, D. M. (2010). pH-(low)-insertion-peptide (pHLIP) translocation of membrane impermeable phalloidin toxin inhibits cancer cell proliferation. Proceedings of the National Academy of Sciences, 107(47), 20246-20250.
- Hunt, J. F., Rath, P., Rothschild, K. J., & Engelman, D. M. (1997). Spontaneous, pH-dependent membrane insertion of a transbilayer α-helix. Biochemistry, 36(49), 15177-15192.
- Palanikumar, L., Al-Hosani, S., Kalmouni, M., Nguyen, V. P., Ali, L., Pasricha, R., Barrera, F.N. and Magzoub, M. (2020). pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Communications biology, 3(1), 95.
- Nguyen, V. P., Alves, D. S., Scott, H. L., Davis, F. L., & Barrera, F. N. (2015). A novel soluble peptide with pH-responsive membrane insertion. Biochemistry, 54(43), 6567-6575.
- Palanikumar, L., Kalmouni, M., Houhou, T., Abdullah, O., Ali, L., Pasricha, R., Straubinger, R., Thomas, S., Afzal, A.J., Barrera, F.N. and Magzoub, M. (2023). pH-responsive upconversion mesoporous silica nanospheres for combined multimodal diagnostic imaging and targeted photodynamic and photothermal cancer therapy. ACS nano, 17(19), 18979-18999.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in