Exploiting the unique properties of tumors to deliver chemotherapeutics to cancer cells
Published in Cancer

Conventional chemotherapeutics suffer from a number of limitations, including poor solubility, short in vivo circulation half-life and lack of specificity [1]. To overcome these limitations, researchers have developed nanocarriers for the safer and more effective delivery of chemotherapeutics to cancer cells. Most nanocarriers ‘passively’ target tumors by utilizing the enhanced permeability and retention (EPR) effect [2]. However, even in high-EPR tumors, less than 1% of passively targeted nanoparticles accumulate in tumors [3]. Alternatively, nanocarriers can be targeted to cancer cells by coating their surface with targeting moieties that bind to overexpressed receptors on the surface of cancer cells [4]. Several issues, however, have restricted the general use of this strategy in cancer therapy as during in vivo blood circulation, the formation of a serum protein corona on a nanocarrier surface can mask the ability of the moiety to interact with cancer cell surface receptors [5]. As a result, we have sought to design a nanoparticle that overcomes these aforementioned issues with current nanocarriers.
A hallmark of many tumors is an acidic microenvironment, which is a consequence of the altered metabolism of cancer cells [6]. This extracellular acidity plays a key role in promoting invasion, proliferation and chemoresistance through increased secretion and activation of proteases, including matrix metalloproteinases (MMPs), bone morphogenetic protein-1-type metalloproteinases, tissue serine proteases, and adamalysin-related membrane proteases [7]. Conversely, the acidity of the tumor microenvironment can be utilized to effectively deliver anticancer drugs to cancer cells. A prime example of this approach is the use of the pH (Low) Insertion Peptides (pHLIPs), which are a unique class of moderately hydrophobic peptides derived from the transmembrane helix C of bacteriorhodopsin [8]. At physiological pH, pHLIPs bind weakly and superficially to membranes in a largely unstructured conformation, whereas under acidic conditions the peptides insert into lipid bilayers forming a transmembrane α-helix [9]. Although pHLIPs have demonstrated pH-dependent membrane insertion and have been successfully used for imaging of cancer cells, these peptides exhibit modest targeting of mildly acidic tumors and have a tendency to aggregate under low pH conditions [10]. The acidity-triggered rational membrane (ATRAM) peptide, a second generation pH-responsive peptide developed by our collaborators, the Barrera lab at the University of Tennessee at Knoxville, is less aggregation prone and has shown superior cancer cell targeting to pHLIPs under the mildly acidic conditions of most tumor microenvironments [11].
As a research group, we are interested in developing novel tumor-specific drug delivery nanoplatforms. Here, we used a simple approach and readily available low-cost materials to prepare biocompatible, biodegradable and economical pH-responsive hybrid nanoparticles for the effective delivery of chemotherapeutics specifically to tumor cells. Thus, unlike many nanocarriers, which require complex chemistry and costly equipment and materials, our nanoparticles can be easily prepared and used by other researchers, even those with limited resources.

Figure 1. NYU Abu Dhabi researchers developed nanocarriers to effectively deliver chemotherapeutics specifically to cancer cells, while minimizing exposure to healthy tissue.
Our hybrid nanoparticles consist of a hydrophobic core wrapped with a protein shell. The core can be loaded with a wide range of chemotherapeutics. The protein shell, which is designed to prolong the blood circulation time, is crosslinked to ensure that the nanocarrier remains stable long enough to reach the target location. Finally, the nanocarrier is functionalized with ATRAM to facilitate uptake specifically in cancer cells within the acidic environment of solid tumors.
As reported in our paper, pH-Responsive High Stability Polymeric Nanoparticles for Targeted Delivery of Anticancer Therapeutics, published this week in Communications Biology, this approach not only prevents targeting of healthy tissues, thereby reducing harmful side effects, but also enhances the potency of the encapsulated drug by accumulating it at the desired site of action.
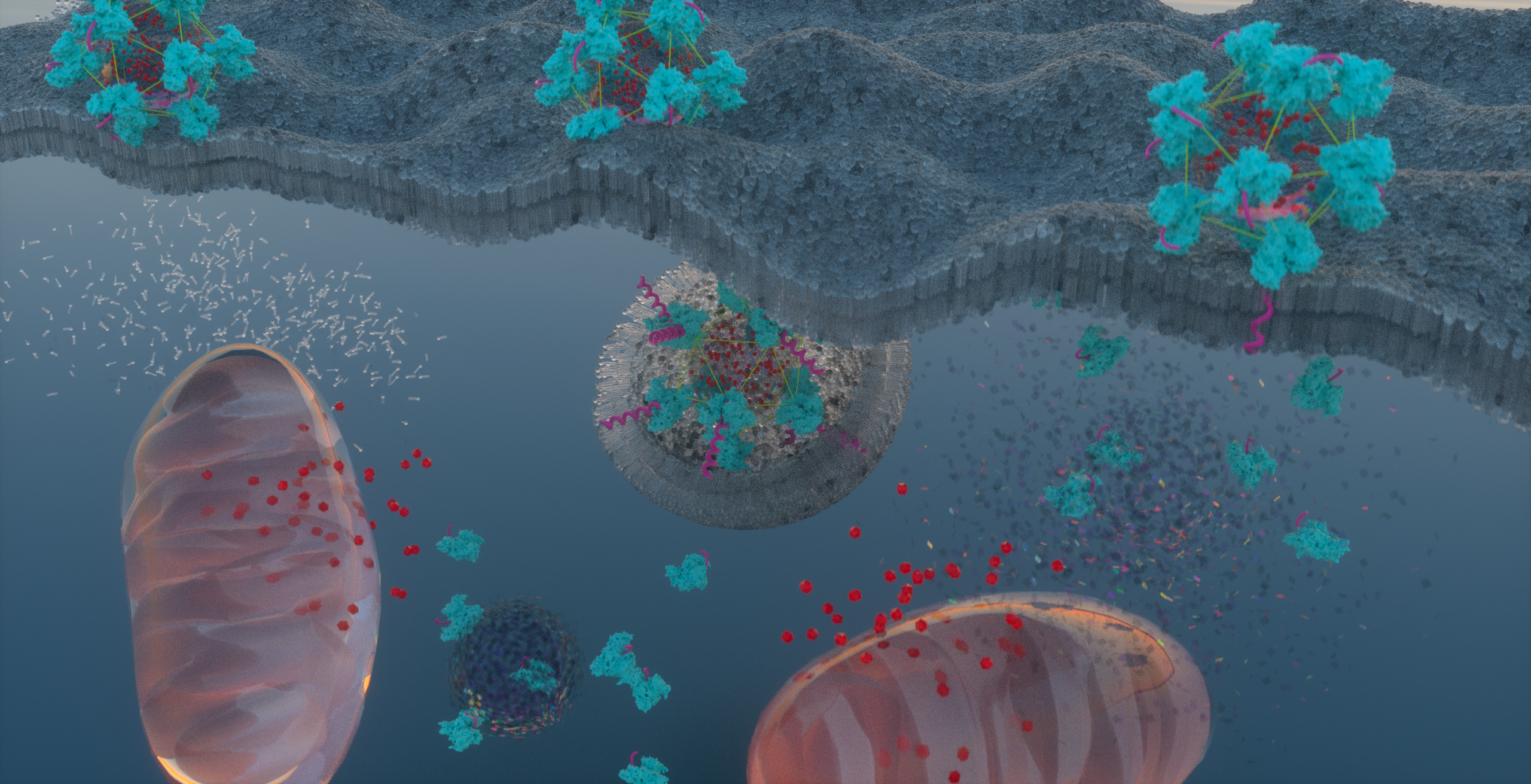
Figure 2. Schematic representation of cellular uptake, by both energy-independent and -dependent mechanisms, of the ATRAM-BSA-PLGA NPs at acidic tumoral pH, followed by intracellular release of the mitochondria-targeting Dox-TPP cargo. Artwork designed by Jumaanah Alhashemi (Assistant Director, Research Visualization and Fabrication Services, NYU Abu Dhabi).
Although this began as a small side-project, and our intention was to publish a communication or short paper reporting the results, by the time it was finally published the paper had grown to include 8 large figures and 20 supporting figures. In particular, the three expert reviewers that evaluated the manuscript homed in on key questions that guided us towards more experiments which, in turn, strengthened our findings. The review process pushed us to further develop additional ideas and experiments that turned this side-project into a research highlight on the journal’s website! Overall, this study is a true example that having a great team, motivation and creativity can lead to great ideas and experiences and we look forward to continuing our work in drug delivery and cancer therapeutics.
Written by Mona Kalmouni, Palanikumar Loganathan, Sumaya Al-Hosani & Mazin Magzoub.
References
1. Torchilin, V., Tumor delivery of macromolecular drugs based on the EPR effect. Advanced drug delivery reviews, 2011. 63(3): p. 131-135.
2. Golombek, S.K., et al., Tumor targeting via EPR: Strategies to enhance patient responses. Advanced drug delivery reviews, 2018. 130: p. 17-38.
3. Nakamura, Y., et al., Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjugate chemistry, 2016. 27(10): p. 2225-2238.
4. Porfire, A., et al., Liposomal Nanoformulations as Current Tumor-Targeting Approach to Cancer Therapy. Liposomes, 2017: p. 245.
5. Oh, J.Y., et al., Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nature communications, 2018. 9(1): p. 1-9.
6. Huang, S., et al., Tumor targeting and microenvironment-responsive nanoparticles for gene delivery. Biomaterials, 2013. 34(21): p. 5294-5302.
7. An, M., et al., pH-(low)-insertion-peptide (pHLIP) translocation of membrane impermeable phalloidin toxin inhibits cancer cell proliferation. Proceedings of the National Academy of Sciences, 2010. 107(47): p. 20246-20250.
8. Andreev, O.A., D.M. Engelman, and Y.K. Reshetnyak, Targeting diseased tissues by pHLIP insertion at low cell surface pH. Frontiers in physiology, 2014. 5: p. 97.
9. Reshetnyak, Y.K., et al., Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane. Proceedings of the National Academy of Sciences, 2008. 105(40): p. 15340-15345.
10. Andreev, O.A., D.M. Engelman, and Y.K. Reshetnyak, pH-sensitive membrane peptides (pHLIPs) as a novel class of delivery agents. Molecular membrane biology, 2010. 27(7): p. 341-352.
11. Nguyen, V.P., et al., A novel soluble peptide with pH-responsive membrane insertion. Biochemistry, 2015. 54(43): p. 6567-6575.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in