Phosphoglycerate kinase 1 acts as a cargo adaptor for EGFR lysosomal transport
Published in Cell & Molecular Biology

Brief Summary
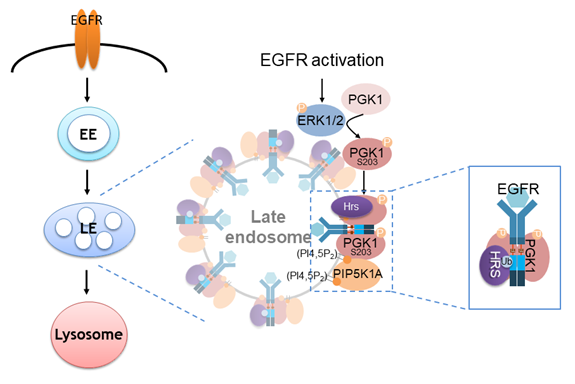
The epidermal growth factor receptor (EGFR) plays critical roles in various cellular processes, such as cell growth and migration. An essential regulatory mechanism for inhibiting EGFR function involves its endocytic transport to the lysosome for protein degradation. Cargo adaptors recognize specific sorting motifs on cargo proteins for proper sorting steps. The dileucine-based sorting motif has been previously identified for the endosomal sorting of EGFR to the lysosome; however, the cargo adaptor recognizing the sorting motif remains unclear. In this study, we discovered that phosphoglycerate kinase 1 (PGK1) is recruited to the endosomal membrane upon phosphorylation, where PGK1 binds to the dileucine sorting motif on EGFR to facilitate the lysosomal transport. Furthermore, we elucidated two mechanisms operating together to promote PGK1 recruitment to the endosomal membrane: a lipid-based mechanism involving phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] and a protein-based mechanism involving hepatocyte growth factor receptor substrate (Hrs). These findings suggest an unexpected role for a glycolytic enzyme and significantly contribute to the mechanistic understanding of EGFR transport to the lysosome.
Systemic Screening for Transport Pathways
Endocytic pathways play important roles in regulating various cellular processes by coordinating the transport of cargoes within the endosomal compartment. Previously, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), a glycolytic enzyme, was identified as an inhibitor of endocytic protein transport during nutrient starvation. This discovery suggested the unexpected role of metabolic enzyme functioning in protein transport other than canonical glycolytic pathway. To understand the involvement of glycolytic enzymes in vesicular transport regulation, we aim to identify new metabolic enzymes involving in protein transport. Through the isolation of a membrane fraction enriched for endosomes from HeLa cells, we discovered PGK1 as another glycolytic enzyme present on the endosomal membrane. Our initial investigation focused on determining the impact of PGK1 on intracellular protein transport pathways. We then took advantage of the quantitative screening of protein transport by tracking the colocalization of model cargoes with specific organelles to characterize the role of PGK1 in different protein transport pathways. Unlike the effect on intracellular transport affected by GAPDH, knockdown PGK1 in cells did not affect ER-to-Golgi anterograde transport, Golgi-to-plasma membrane anterograde transport, Golgi-to-ER retrograde transport, endocytic retrograde transport of cholera toxin B-subunit to the Golgi, and endocytic recycling of transferrin receptor back to the plasma membrane. Strikingly, we found the delay of endocytic transport of EGFR to the lysosome in cells treated with siRNA against PGK1, suggesting that PGK1 promotes EGFR endosomal trafficking. Consistently, we also demonstrated that knockdown PGK1 in cells also prevented the degradation of EGFR upon EGF stimulation, indicating that PGK1 facilitates the EGFR lysosomal transport for protein degradation.
PGK1 Promotes EGFR Lysosomal Transport Independent of Enzymatic Activity
PGK1 not only catalyzes the conversion of 1,3-bisphosphoglycerate (1,3-BPG) to 3-phosphoglycerate (3-PG) and adenosine triphosphate (ATP) but also acts as a protein kinase to regulate mitochondria function and autophagy. We then sought to identify whether the kinase activity of PGK1 is involved in EGFR protein degradation. After treatment with siRNA against PGK1, we introduced wild-type PGK1 and kinase-dead mutant form PGK1-T378P and found both forms showed similar patterns of EGFR degradation and EGFR lysosomal trafficking upon EGF stimulation. Thus, PGK1 regulates EGFR lysosomal degradation independent of its kinase activity.
PGK1 Acts as a Cargo Adaptor
As further evidence in favor of PGK1-mediated EGFR degradation in a kinase-independent mechanism, we then sought to understand how PGK1 promotes EGFR lysosomal trafficking and degradation in cells. Cargo sorting involves protein factor binding to the cytoplasmic domain of cargo proteins for the transport of cargo proteins to the membrane compartment. We first tested whether PGK1 interacts with EGFR. EGFR is a single transmembrane protein with three prominent cytoplasmic domains, the juxtamembrane region, kinase domain, and cytoplasmic tail. We then appended each fragment to glutathione S-transferase (GST) and examined which domain bound directly to soluble PGK1 in a pull-down assay. Strikingly, we detected the direct interaction between PGK1 and EGFR juxtamembrane region. We then performed alanine-scanning mutagenesis on residues inside the juxtamembrane construct and examined their interaction with PGK1. Among them, only Leu679 and Leu680 were found critical for binding to PGK1. Leu679 and Leu680 were reported to conform to a dileucine-based sorting motif for efficient lysosomal transport of EGFR. Consistent with these findings, PGK1 does not associate with mutant EGFR (EGFR-L679AL680A). We also mutated the dileucine motif in the full-length EGFR in cells and observed the defect of EGFR degradation upon EGF stimulation. The resulting mutant EGFR, compared with wild-type EGFR, shows less localization to the lysosome in EGF-treated cells.
Conclusion & Significance
In summary, we have discovered a metabolic kinase PGK1 moonlighting as an effector in an intracellular transport pathway, whereby PGK1 functions as a cargo adaptor for EGFR lysosomal trafficking. In the case of EGFR, it has been known that cargo sorting for its lysosomal targeting and protein degradation are essential machinery to regulate EGFR signaling. Previous findings also revealed that the dileucine motif on EGFR (Leu679 and Leu680) facilitates the incorporation of ligand-receptor complex into lysosomal membrane compartment for protein degradation. In our current model (see the figure below), we have uncovered that PGK1 is being recruited onto the endosomal membranes. We have also identified the cargo adaptor function of PGK1 by showing that the dileucine motif in the EGFR juxtamembrane region recognized by PGK1 constitutes sorting signals to promote lysosomal trafficking.
Our discovery unravels the understanding of EGFR transport to the lysosome in multiple ways. We suspect that other endocytic proteins would also require both ubiquitin-dependent and ubiquitin-independent mechanisms working in concert to achieve efficient targeting to the lysosome.
References
-
Chu, S.L. et al. Phosphoglycerate kinase 1 acts as a cargo adaptor to promote EGFR transport to the lysosome. Nat Commun 15, 1021 (2024).
-
Hsu, J.W. et al. The protein kinase Akt acts as a coat adaptor in endocytic recycling. Nat Cell Biol 22, 927-933 (2020).
- Yang, J.S. et al. GAPDH inhibits intracellular pathways during starvation for cellular energy homeostasis. Nature 561, 263-267 (2018).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in