Photosensitizer-free Visible-light-promoted Glycosylation Enabled by 2-Glycosyloxy Tropone Donors
Published in Chemistry
Photochemical glycosylation has attracted considerable attention in carbohydrate chemistry. Currently, visible-light-induced glycosylation reactions are achieved by either photoactivating a photosensitizer or using a stoichiometric activator first to generate an intermediate, which facilitates the departure of the leaving group, forming the glycosyl cation (Figure 1a). However, to the best of our knowledge, visible-light-promoted glycosylation via a photoactive glycosyl donor has not been reported. Thus, we directed our efforts toward designing a photoactive-leaving group to fill this gap. The capacity of the chromophoric leaving group to absorb light at the irradiation wavelength (preferably visible light) is one of the fundamental prerequisites for a photoactive glycosyl donor. Through literature research, we understand that tropolone and its derivatives are a class of non-benzenoid aromatic compounds known for their diverse biological activities, and exhibits fluorophoric properties. In addition, tropolonate salts are suitable for acyl-transfer catalysts under visible-light conditions (Figure 1b, ACS Catal. 2020, 10, 12596-12606). Based on the properties of tropolone mentioned above, we envisaged that tropolone might be an ideal photoactive-leaving group for visible-light-induced glycosylation (Figure 1c).
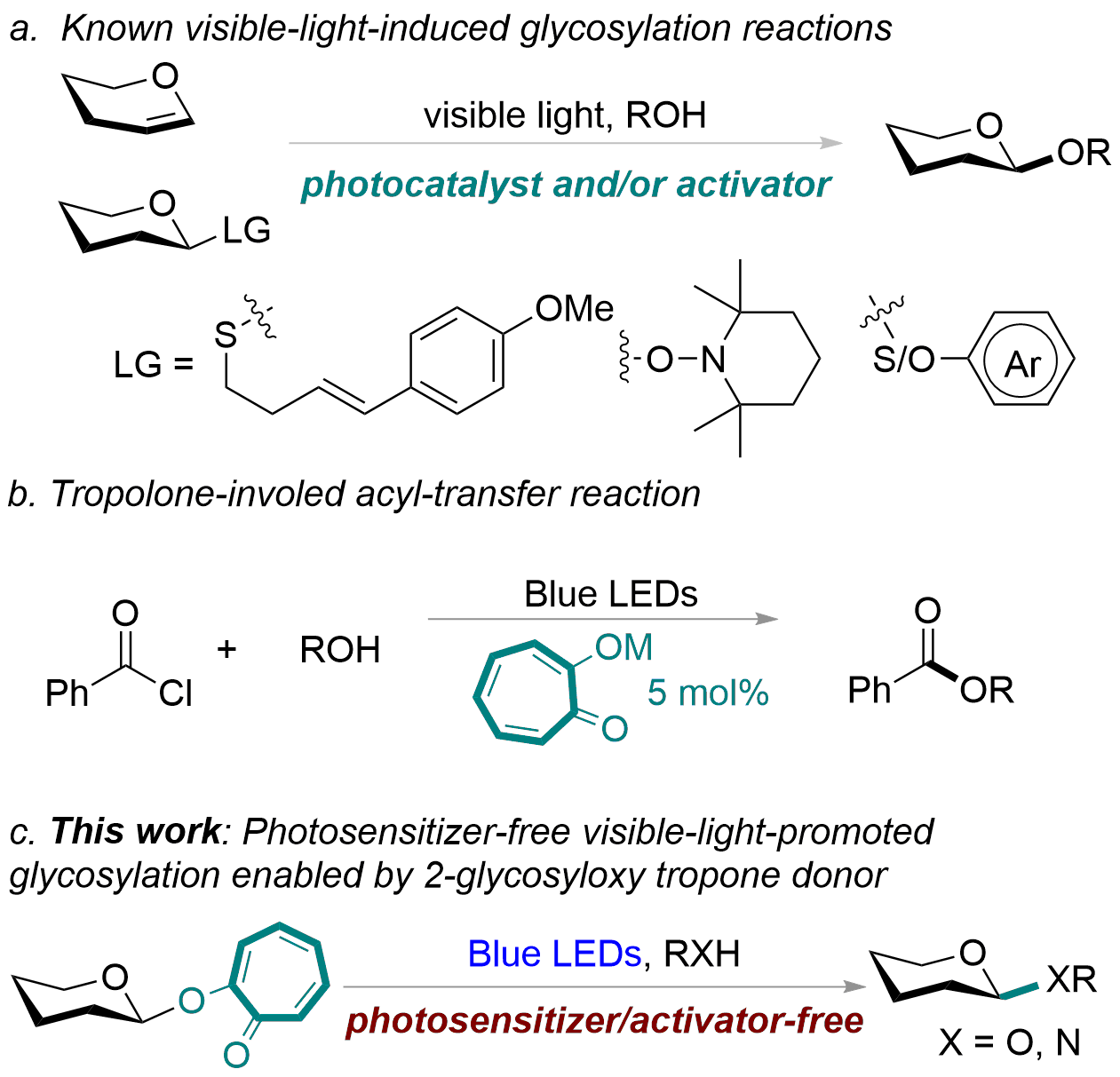
Figure 1. Background and this work.
In our study, this glycosylation reaction proceeds at ambient temperature to give a wide range of O-glycosides or oligosaccharides. Primary, secondary, and tertiary alcohols are suitable glycosyl acceptors under the standard reaction conditions, resulting in excellent yields of the desired glycosides, and the reaction of perbenzoylated 2-galactosyloxy tropone with various glycosyl acceptors bearing a free hydroxyl group at C-6, C-2, C-3, and C-4 positions generated the disaccharides with yields ranging from 88% to 96%. The utilities of this method are also highlighted by the construction the stereoselective preparation of glycosyl phosphosaccharides. β-Glycosyl phosphosaccharides were efficiently synthesized with moderate to good yields through glycosylation reaction of perbenzoylated 2-glycosyloxy tropone donor with various phosphate acceptors. The exposure of perbenzylated 2-glycosyloxy tropone donor and phosphate nucleophiles to visible-light irradiation led to the glycosyl phosphosaccharides with high α-stereoselectivity. This method was further demonstrated through N-glycosylation, which is another important aspect of evaluating the effectiveness of a new glycosyl donor. Pyranosyl-/Furanosyl-donors effectively reacted with pyrimidines or purines to form the corresponding N-glycosides with excellent yields (Figure 2).
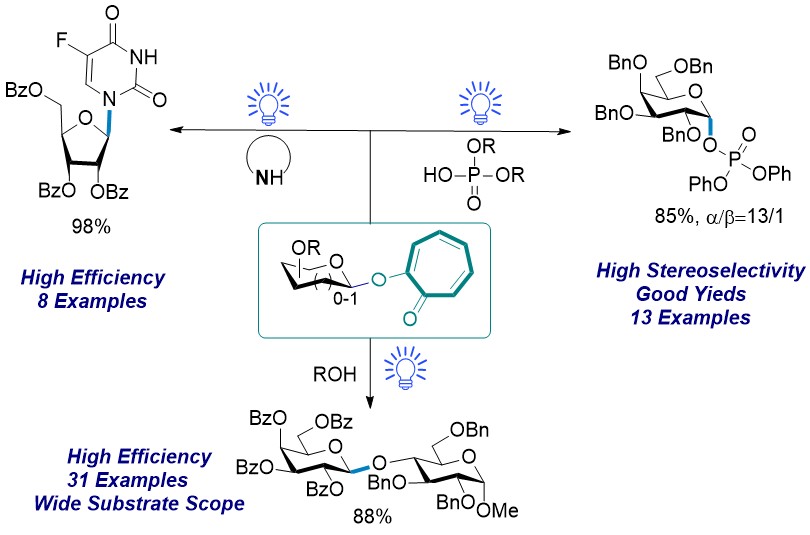
Figure 2. Scope of the substrates for O-glycosylation, N-glycosylation and the stereoselective preparation of glycosyl phosphosaccharides.
The late-stage glycosylation of natural products or pharmaceuticals on gram scales are also feasible under this method (Figure 3a). The ultimate aim of a glycosylation method is to synthesize glycans. Thus, a hexasaccharide was prepared via iteratively visible-light-promoted glycosylation (Figure 3b).
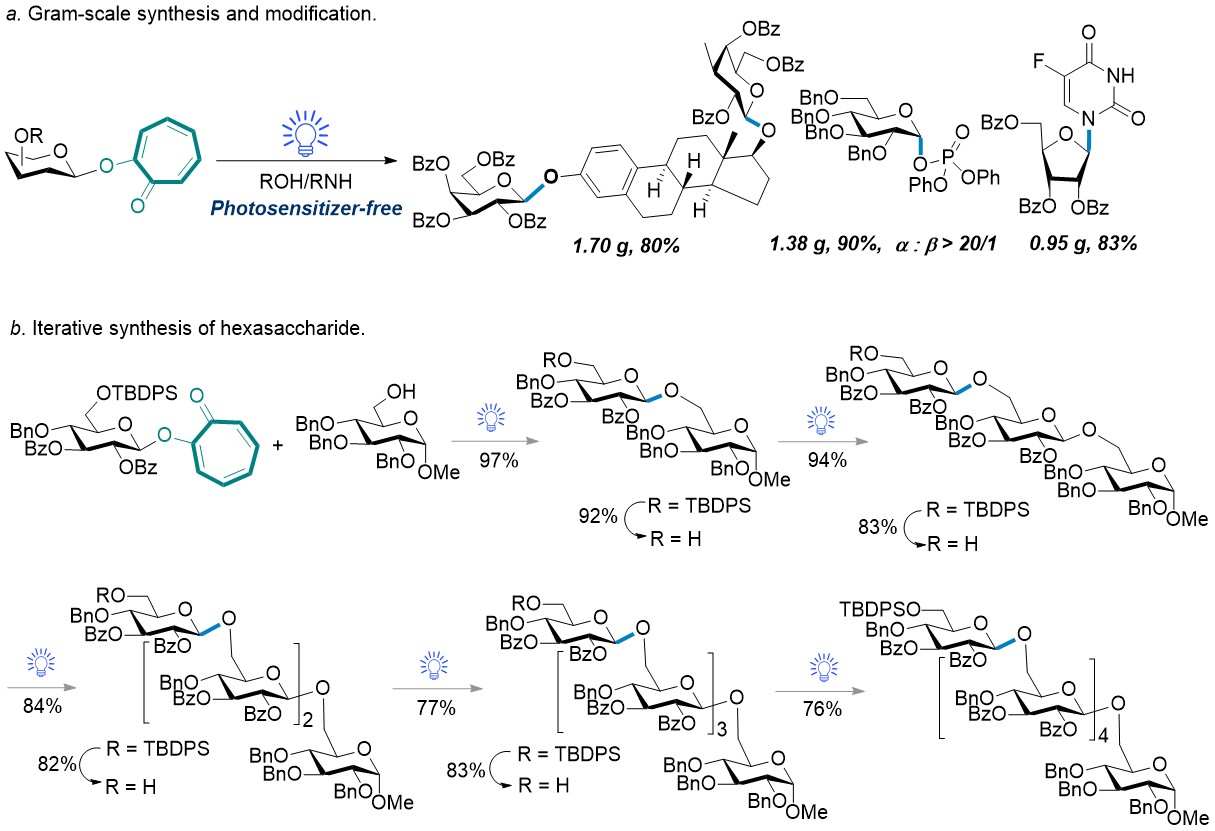
Figure 3. (a) Gram-scale synthesis and modification. (b) Iterative synthesis of hexasaccharide.
Ultimately, we developed a versatile visible-light-driven glycosylation method using the 2-glycosyloxy tropone donor. The efficiency, robustness, and selectivity of the presented method make it an attractive protocol for synthesizing glycans and glycoconjugates.
More details of this work could be found here: “Photosensitizer-free Visible-light-promoted Glycosylation Enabled by 2-Glycosyloxy Tropone Donors” in Nature Communications.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in