Potent induction of humoral and cellular immunity after bivalent BA.4/5 mRNA vaccination in dialysis patients
Published in Bioengineering & Biotechnology, General & Internal Medicine, and Immunology

Background
I am a PhD student in the Transplant and Infection Immunology lab led by Professor Martina Sester. When I started my PhD, the lab group had already jumped on the SARS-CoV-2 research bandwagon for over 2 years. In the beginning of the pandemic, they characterized SARS-CoV-2–specific T cells and antibodies of patients with different COVID-19 disease severity compared to uninfected controls1. After licensing of vaccines, the focus of research shifted to the characterization of vaccine-induced immunity. In a first observational study, the immunogenicity and reactogenicity of different vaccine regimen were compared2. Additionally, the focus was also directed towards SARS-CoV-2-specific immunity of high-risk patients, such as solid transplant patients3. These numerous studies formed the basis of my project, as ethics applications and declarations of consent had already been submitted and the laboratory procedures had been established.
Why dialysis patients?
Patients with end-stage chronic kidney disease and on dialysis had a higher risk of developing severe COVID-19 disease with a fatal outcome4. This risk has already been minimized by basic immunization but several studies have shown that dialysis patients developed an inadequate humoral and cellular immune response after COVID-19 vaccination5 which waned rapidly6 (Figure 1A). In 2022, the immune-escaping omicron subvariants of concern led to an increased incidence of breakthrough infections in patients and immunocompetent individuals. To protect this high-risk group, more frequent booster vaccinations, such as the currently licensed bivalent mRNA vaccine targeting both the parental strain and the Omicron variant BA.4/5, were recommended to be administered in order to achieve a similar level of immunity as in immunocompetent individuals. This was one of the major reason for us to focus on dialysis patients and on the immunogenicity after bivalent BA.4/5 vaccination in regard to different research questions (Figure 1B).
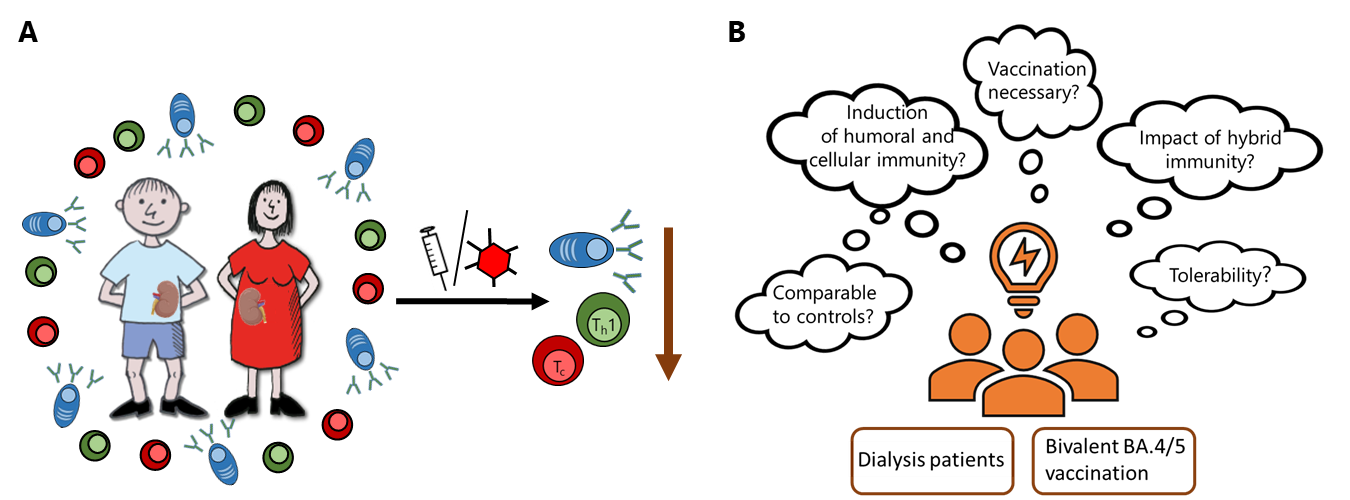
Collaboration is the be-all and end-all
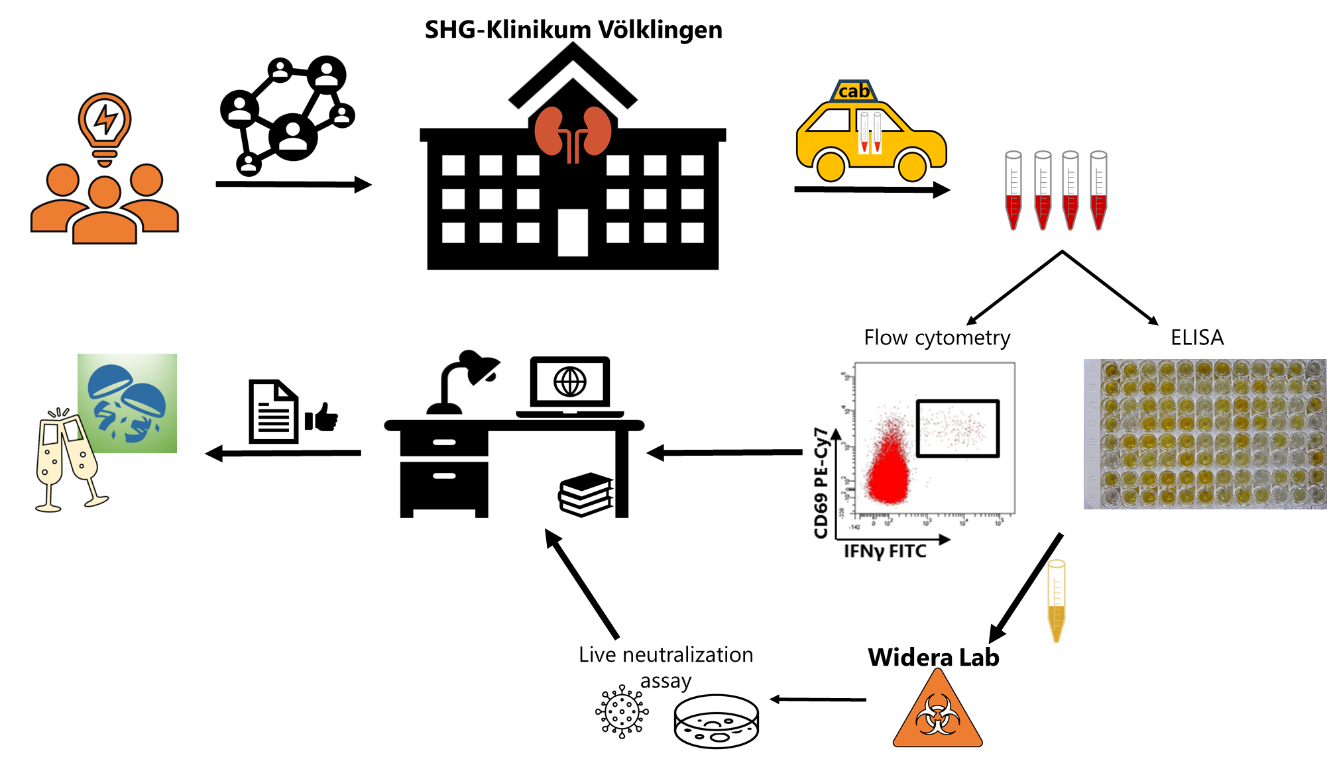
What comes next (Figure 2)? As my supervisor wants to say: “Build a network! Establish infrastructure or use existing infrastructure!” My supervisor's husband is principal consultant of nephrology at the SHG clinics in Völklingen, so the decision to ask him to work with me was quickly made. After talking to him about our research project, he was immediately on board. But the most important statement during the conversation was: "The key to collaboration is presence". So, my first act was to travel to Völklingen and introduce myself to the senior consultant (a former MD student of the lab), the physicians, and nurses. Just as important as the introductory round, however, is a discussion of the project and, above all, the most important barriers:
- Blood sampling should be performed prior to dialysis
- Blood transport from Völklingen to Homburg (50 km distance). A special thanks to “taxi principal consultant”
- Collection of questionnaire to vaccination and infection history and vaccine-induced adverse events
- Major Problem: Vaccine hesistancy
Now the project can begin!
In our observational study, we recruited 33 dialysis patients and 58 immunocompetent controls of which blood samples were collected before and 13-18 days after vaccination. By flow cytometry and ELISA, we determined specific humoral and cellular immunity toward the spike protein derived from the parental SARS-CoV-2 strain as well as from the Omicron variants BA.1, BA.2 and BA.4/5. The flood of samples was one of the biggest challenges. SARS-CoV-2, like influenza, occurs preferentially in the fall and as the variant-adapted vaccines were available, all vaccination enthusiasts were vaccinated in one package. During the running experiments, our interest in neutralizing antibodies became stronger. A quick request to Marek Widera from Frankfurt University and we were able to send the serum samples to the Widera lab to determine neutralization capacity.
The successful collaboration with clinical departments as well as other scientific laboratory groups made this study feasible and allowed us to achieve interesting and important results:
- Bivalent BA.4/5 mRNA vaccine induces humoral and cellular in patients and to a similar extent as in controls.
- Neutralizing antibody activity towards the parental strain was higher compared to Omicron subvariants, whereas T-cell levels towards parental strain and Omicron subvariants were of similarly high magnitude.
- Unlike in controls, previous infection of patients was even associated with higher levels of spike-specific CD4+ T cells.
Take home message
Infections, regardless of their severity, boost immunity to a higher degree than vaccinations alone. Nevertheless, immunocompromised patients with a high risk of severe disease and a faster loss of specific immunity after vaccination should be made aware of the current recommendations for annual vaccinations so that infections can be survived well in the future. These findings will hopefully serve to reduce the ever-increasing vaccination fatigue.
References
- Schub, D., et al. High levels of SARS-CoV-2-specific T cells with restricted functionality in severe courses of COVID-19. JCI Insight 5(2020).
- Schmidt, T., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med 27, 1530-1535 (2021).
- Schmidt, T., et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am J Transplant 21, 3990-4002 (2021).
- Era-Edta Council & Eracoda Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 36, 87-94 (2021).
- Imhof, C., et al. SARS-CoV-2 Spike-specific IFN-gamma T-cell Response After COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant. Transplant Direct 8, e1387 (2022).
- Karakizlis, H., et al. Immunogenicity and reactogenicity of homologous mRNA-based and vector-based SARS-CoV-2 vaccine regimens in patients receiving maintenance dialysis. Clin Immunol 236, 108961 (2022).
Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: Feb 19, 2026
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in