Probing the depths of complex electron shells

Exploring the actinide elements pushes the boundaries of the periodic table. Sitting at the bottom of it, these fascinating elements are the least understood and yet the most intriguing in the chemical world. Their rich chemistry has puzzled scientists for decades, with a variety of oxidation states leading to unusual and diverse bonding behaviors as a result of the gradual filling of their 5f electron shells. In particular, the identification of exotic oxidation states of actinides has long been a challenge for the scientific community, especially for low-valent systems due to their instability. At the same time, the nature of the actinide bond in 5f electron systems remains a hot and sometimes controversial topic in actinide chemistry.
The story described in our Nature Communications paper began more than 10 years ago. We were looking for low-valent uranium compounds, which contain more electrons than other uranium-containing materials. Studying their electronic structure would shed light on the fundamental physics and chemistry of actinide systems. One of the main challenges with such compounds is that they are less stable than other uranium-containing materials and require carefully controlled conditions during synthesis and data collection. The experiments succeeded when our team, based at the Rossendorf beamline of the European Synchrotron Radiation Facility (ESRF, France) and led by Prof. Kristina Kvashinina, joined forces with Prof. Florian Kraus from Philipps-Universität Marburg (Germany). The samples, synthesized in the chemistry laboratory of the Philipps-Universität Marburg, had to be transported to the ESRF under carefully controlled conditions to ensure their stability. To keep them stable, they were sealed under anoxic conditions and transported to the beamline under liquid nitrogen. The experiments were carried out at extremely low temperatures in a fully equipped beamline designed to handle radioactive elements. Finally, using advanced X-ray spectroscopic methods—resonant inelastic X-ray scattering (RIXS) and X-ray absorption near-edge structure in the high-energy resolution fluorescence detection mode (HERFD-XANES)—we were able to directly detect the trivalent oxidation state of uranium (UIII).
These advanced experimental techniques were found to be not only highly sensitive in detecting the UIII oxidation state (by looking at the shift of the main spectral feature), but also capable of providing detailed information about the electronic structure around the uranium atom. The physical process behind the RIXS method involves the excitation of a core electron by the absorption of an X-ray (see Figure 1). To probe the 5f states, the incoming X-ray energy was selected at the uranium M4-edge (core electron in the 3d3/2), and an X-ray spectrometer allowed us to obtain high-resolution data by recording the fluorescence emitted in a specific decay channel. Using cutting-edge electronic structure calculations, we were able to go one step further and provide insights into the actinide bonding debate. Our study presents a systematic investigation of UIII and UIV halides, namely UX3 and UX4 (X = F, Cl, Br, I), and one of the key questions addressed is the following: how ionic are the bonds in these compounds, and what is the behavior of the 5f electrons?
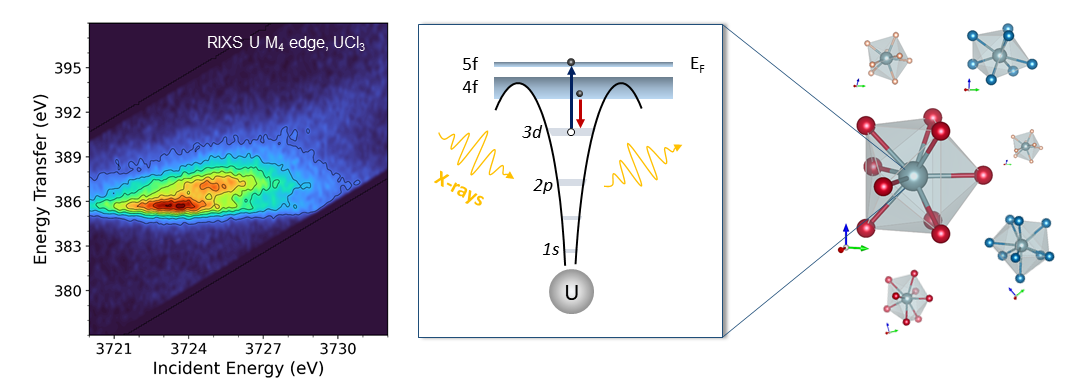
In low-valent uranium compounds, the 5f electronic structure is not expected to contribute significantly to the bonding, and one would rather expect the uranium M4 edge HERFD-XANES to be insensitive to the system-specific stereochemistry, in analogy to the M4,5 edge XANES of their trivalent lanthanide analogues. Contrary to expectations, spectral changes are observed within each series. The UX4 and UX3 data reveal a degree of 5f sensitivity to the specific local environment. Interestingly, the results of the calculations highlight that in low-valent uranium compounds the 5f electrons feel the local environment because it modulates their degree of localization. Our study revealed this surprising sensitivity of uranium's 5f electrons to changes in their local environment, with the interactions between them affecting the ionic character of the uranium-halide chemical bond. These findings challenge existing theories and open up new avenues of research in actinide chemistry.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in