Reimagining antivenoms: from horse serum to biotechnology
Published in Bioengineering & Biotechnology and General & Internal Medicine
The sun sets over a village in sub-Saharan Africa. A child runs barefoot across the dusty ground, laughter echoing in the warm evening air. In the tall grass nearby, a snake waits unseen — until, in an instant, fangs strike skin. Venom spreads through the body. Panic follows. In places like this, far from hospitals and where treatment is scarce, a single bite can mean the difference between life, losing a limb, or even death.

Golden Sentinel: An African cobra (Naja nivea) poised in its native scrubland, displaying its brilliant yellow hood and watchful stance in the midday sun.
Credit: Wolfgang Wüster
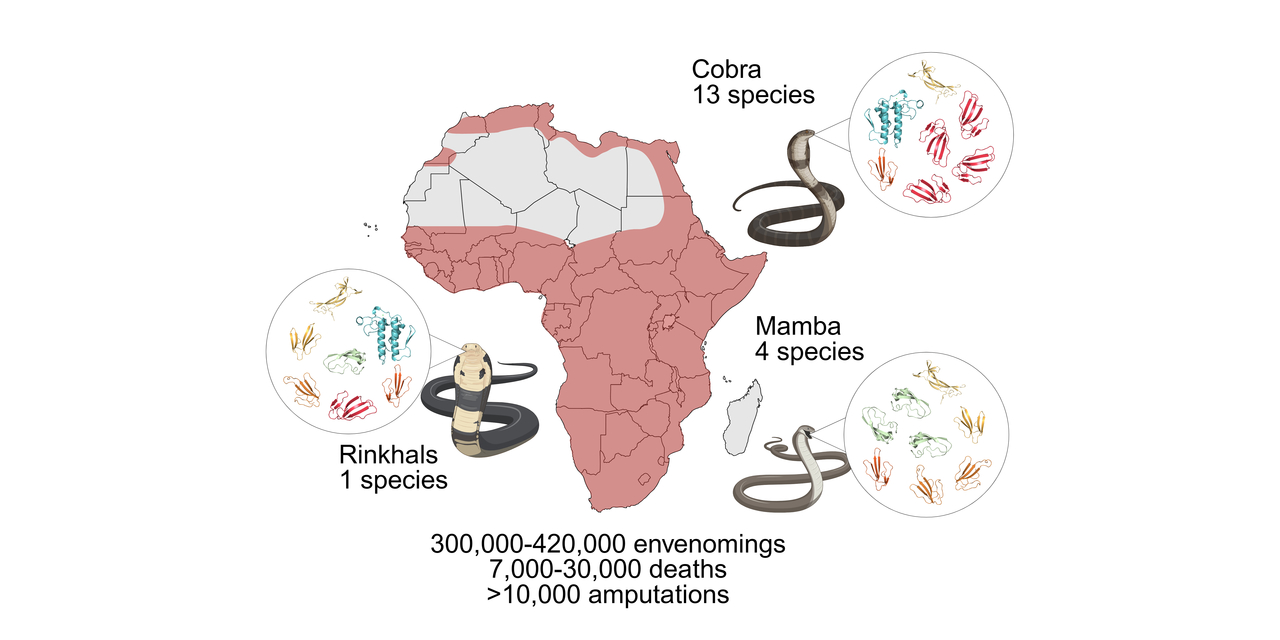
Snakebite envenoming is not just a medical emergency; it’s one of the world’s most neglected health crises. Among the 21 neglected tropical diseases (NTDs) recognized by the World Health Organization, snakebite alone kills more than all the other 20 NTDs combined. Each year, 2.5 million people are bitten, causing over 100,000 deaths and several hundred thousand victims with lost limbs or other permanent disabilities. Yet, antivenom production has barely evolved in over a hundred years, still completely relying on immunized horse serum. These life-saving serums are costly, can cause dangerous allergic reactions, and too often don’t protect against bites from a wide range of snake species. Existing antivenoms also often fail to prevent venoms from causing local tissue damage, which leaves many survivors disabled for life.
Because of the enormous unmet medical need related to snakebite envenomings, we asked ourselves if modern biotechnology could help bring about a new generation of therapies that are safer, cover a wider range of snake species, and are affordable to the very communities most at risk?
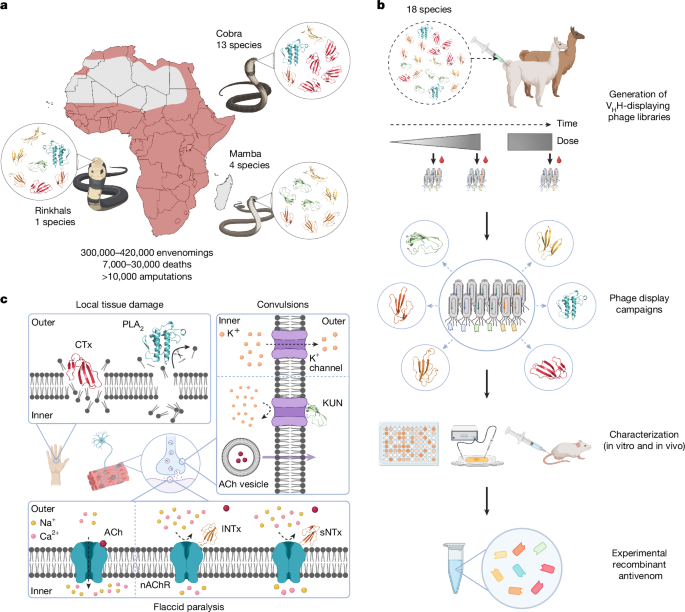
Our study published in Nature provides the answer to exactly this question. By combining just eight nanobodies (tiny antibody fragments derived from alpacas and llamas), we created a recombinant antivenom with continent-wide coverage protecting against 17 of Africa’s 18 most dangerous cobras, mambas, and rinkhals. In preclinical studies, this carefully designed nanobody mixture not only prevented death but also reduced the severe tissue damage that often leads to amputation. These results challenge a century of assumptions that broad protection against multiple snake venoms could not be achieved using something simpler than horse-derived serum. We now know that these assumptions were not correct!
Moments of discovery
Our therapeutic development strategy was to first map which toxins are the medically most relevant ones and then to apply phage display technology to find nanobodies that bind not only one but multiple similar toxins from the venoms of different snake species. We tested more than 1500 nanobodies before we narrowed down to those that showed the strongest and broadest binding to multiple toxins from the same family. Thereafter, we evaluated the most promising nanobodies in vitro and in vivo to find the most broadly neutralizing ones. Based on these results, we designed a mixture of eight nanobodies that we believed had a fair shot at neutralizing a remarkably wide range of venoms.
Our big eureka moment came during the in vivo experiments. Mice that would otherwise have succumbed to lethal doses of venoms survived when treated with the nanobody mixture, often showing none or only minor transient signs of toxicity. In addition, when challenged with cytotoxic venoms that typically cause severe tissue damage, animals treated with the recombinant antivenom displayed substantially reduced tissue damage. These results gave us hope that, in the future, amputations that are often required due to severe tissue damage can be avoided with optimal recombinant antivenom therapy.
Beyond its proven efficacy, our nanobody-based antivenom also holds promise for being safer and more consistent than traditional horse-derived products. Nanobodies themselves have an excellent safety profile, and because they are produced recombinantly, every batch can be made under controlled conditions, eliminating the batch-to-batch variation that can be observed for horse-derived antivenoms. In addition, nanobodies can be manufactured in microbial systems, allowing large-scale, cost-efficient production in bioreactors. Together, these advantages could make future antivenoms more affordable and accessible to the low-resource regions where they are needed most.
Engineering without borders
For us scientists, some of the most rewarding moments were not just seeing the exciting data itself, but the sense of a globally collaborative achievement. Our colleagues in Mexico and the United States carried out the demanding in vivo experiments, showing that the nanobody mixture could protect mice from dying even after being exposed to lethal venom doses. In the UK, other collaborators performed the in vivo experiments that gave us key insights into how well the treatment prevented the devastating tissue damage that often follows snakebites. At the same time, our industry collaborators contributed by running in vitro neutralization assays to reduce the number of mice needed for in vivo experiments and providing complementary evidence of broad protection at a molecular level. To understand exactly how the nanobodies neutralized the toxins, we also determined the molecular structure of some nanobody:toxin complexes with colleagues from both Denmark and the US. In many ways, the science advanced because scientists from over 15 countries and different disciplines came together to solve a global problem that none of us could have tackled alone.
The slithering road ahead
Still, there are important challenges ahead. Translating a preclinical proof of concept from mice into a clinically approved therapy is a long and complex journey. Nanobodies typically have a short half-life in circulation, which might necessitate high doses and/or repeated dosing. Therefore, strategies for extending their half-life may be needed. The timing of treatment also matters; in real-world settings, patients often arrive at the hospital hours after a bite, and we need to understand how effective the antivenom remains under those circumstances. Further, manufacturing at scale, ensuring stability in tropical climates, and navigating regulatory pathways are also critical challenges that must be addressed before a recombinant antivenom product can reach patients.
Despite these challenges, we believe this work represents a new way of thinking about antivenoms and potentially about therapies for other diseases driven by diverse and complex disease targets. It shows that with rational design and strong collaboration, it is possible to simplify and to distill protection against snakebite envenoming into a defined set of molecules rather than rely on the uncontrolled complexity of serum from immunized animals.
A vision for the future
Our ultimate goal is clear! We wish to create a new generation of antivenoms that are safer, more effective, and affordable for the people who need them most. The eight-nanobody mixture described here is only a first step towards that goal, but it demonstrates that modern biotechnology has the potential to serve the world’s most neglected victims.
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in