Retooling NK cells to enhance HIV vaccine efficacy
Published in Bioengineering & Biotechnology, General & Internal Medicine, and Immunology
Despite over forty years of research and significant progress in HIV treatment and prevention, a safe and widely effective HIV vaccine remains one of the greatest unmet needs in global health. Tools such as antiretroviral therapy (ART), pre-exposure prophylaxis (PrEP), and regular testing have helped reduce transmission rates, but these strategies rely on consistent use and long-term access. To truly end the epidemic, we still need a vaccine that is safe, durable, and accessible to all. Traditional HIV vaccine design primarily focuses on generating robust antibody and T-cell responses, relying heavily on the adaptive immune system. However, a growing body of research suggests that this conventional approach may overlook the untapped potential of the innate immune system, particularly natural killer (NK) cells. These fast-acting immune cells play a critical role in the body's first line of defense against viral infections and tumors and are increasingly recognized as key contributors to effective vaccine-induced immunity.
Among their many roles, NK cells can participate in antibody-dependent cell-mediated cytotoxicity (ADCC), a process in which antibodies bind to infected cells and "flag" them for elimination by NK cells. This function is of particular interest in HIV vaccine research, as ADCC has emerged as a potential immune correlate of protection. Notably, the landmark RV144 trial—conducted in Thailand and the first to demonstrate modest efficacy (31%) in reducing HIV acquisition—suggested that vaccine-induced ADCC may have contributed to the observed protection. These findings opened the door to considering how NK cells could be manipulated to boost vaccine effectiveness. Building on this concept, our recent study published in npj Vaccines explored this idea further. The study, titled "Novel oral adjuvant to enhance cytotoxic memory-like NK cell responses in an HIV vaccine platform," investigates a new approach to enhancing NK cell function as part of an HIV vaccine regimen.
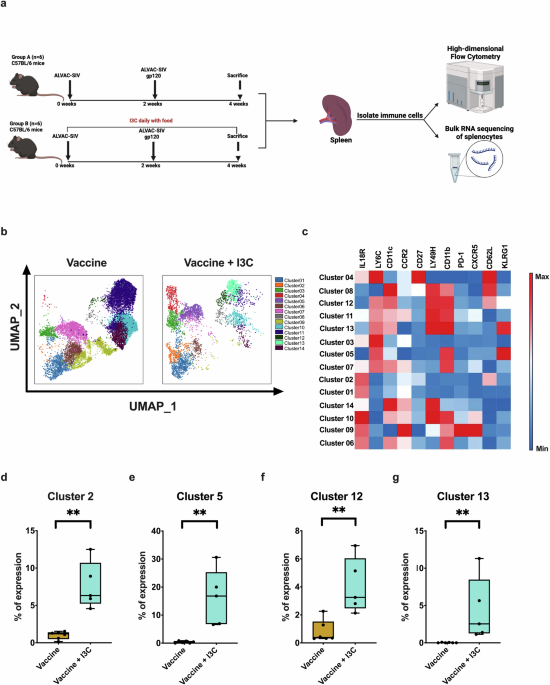
Although Aryl hydrocarbon receptor (AHR) agonists have been extensively studied in cancer and autoimmune diseases, their role in antiviral immunity—particularly in shaping NK cell responses—has remained relatively underexplored. Our study sought to bridge this knowledge gap and determine whether oral indole-3-carbinol (I3C), an AHR agonist, could enhance the effectiveness of an RV144-like vaccine platform through its interaction with the AHR pathway. To test this hypothesis, we used a preclinical mouse model designed to replicate the RV144 vaccine platform, which combines a live attenuated viral vector (ALVAC) with a recombinant HIV envelope protein (gp120). I3C was administered orally alongside the vaccine to evaluate its ability to enhance immune responses.
We found that mice receiving I3C had a greater number of NK cells expressing KLRG1 receptors, typically found on mature, highly cytotoxic NK cells with “memory-like” behavior. These KLRG1⁺ NK cells demonstrated greater cytotoxic responses when re-exposed to vaccine antigens and revealed a greater potential to work more effectively in coordination with antibodies and T cells induced by the vaccine. Importantly, our epigenetic analysis suggested that I3C may trigger long-lasting modifications in NK cells, potentially laying the foundation for durable immunity, an especially valuable feature in HIV vaccine development, where long-term protection is essential. The ability of NK cells to "remember" previous encounters with antigens challenges the long-standing belief that only B and T cells possess immune memory, and this insight may significantly alter how we think about durable vaccine protection. Beyond their cytotoxic activity, NK cells also influence the broader immune environment by secreting cytokines such as interferon-gamma (IFN-γ), which help shape adaptive immune responses. By enhancing NK cell activity through AHR activation, I3C may promote a more coordinated, multi-layered immune response—one that effectively bridges the innate and adaptive arms of the immune system.
.jpeg)
Expansion of vaccine antigen-specific KLRG1+ NK cells may occur through AhR agonist-mediated epigenetic modifications.
Our findings underscore the importance of broadening the scope of immune targets in HIV vaccine research. Rather than focusing exclusively on eliciting neutralizing antibodies or cytotoxic T-cell responses, next-generation vaccines might be designed to also activate NK cells as part of a comprehensive immunological strategy. This integrated approach could amplify vaccine efficacy, increase durability, and provide better protection against diverse HIV strains. While more research—including human trials—is needed, this work offers a new direction that combines the innate immune system with classical vaccine design. It also supports the growing understanding that the innate immune system is not just a blunt, short-term defense but a flexible and trainable part of our immunity. Moreover, our work contributes to the expanding recognition that innate immunity is not merely a non-specific defense mechanism but a dynamic and adaptable system that can be trained, enhanced, and strategically deployed. As we continue to uncover how NK cells function in the context of vaccination, we may find new ways to design vaccines that offer stronger, longer-lasting, and more equitable protection.
Follow the Topic
-
npj Vaccines

A multidisciplinary journal that is dedicated to publishing the finest and high-quality research and development on human and veterinary vaccines.
Related Collections
With Collections, you can get published faster and increase your visibility.
Therapeutic HPV vaccines
Publishing Model: Open Access
Deadline: Jun 30, 2026
Lipid nanoparticle (LNP)-adjuvanted vaccines
Publishing Model: Open Access
Deadline: May 19, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in