Rotten to the core: a neurofunctional signature of subjective disgust generalizes to oral distaste and socio-moral contexts
Published in Ecology & Evolution, Neuroscience, and Protocols & Methods

Disgust represents an evolutionary adaptive protective response and is present in many aspects of our daily life. For example, people may feel disgusted when they drink or eat something distasteful (‘distaste’), see poor hygiene or rotten food (‘core disgust’) or witness someone violating social and moral norms (‘socio-moral disgust’). Evolutionary and disgust appraisal models have long postulated an association between these processes and hypothesized that the subjective experience of core disgust has evolved from the basal distaste response and later been adopted into the social moral domain1-3. However, a neural evaluation of this hypothesis was precluded by the lack of a precise neurobiological marker that tracks subjective disgust experience in humans.
The neural representation of disgust is inter-related with the brain basis of other defensive responses, for instance, fear4 or also general negative affect, however, the shared and separable neurofunctional representations underlying these negative emotions have not been systematically investigated.
We here capitalized on recent methodological advances in multivariate pattern analysis based neural decoding techniques and fMRI to develop an accurate and generalizable whole-brain signature predictive of momentary self-reported subjective disgust experience to address these questions.
A sensitive and comprehensive brain signature for subjective disgust experience
The experience of disgust was induced by visual core disgust stimuli with varying levels of subjective disgust induction e.g., by stimuli showing disgusting situations (Figure 1) while brain activity was recorded using fMRI. To facilitate robust results, we employed a discovery (n=78) and pre-registered validation (n=30) cohort design. Participants were instructed to react naturally to the stimuli and report their momentary level of disgust during each trial on a 5-point Likert scale ranging from 1 (neutral/slightest disgust) to 5 (very strong disgust).
Next, utilizing machine-learning-based neural decoding, we established and validated a comprehensive neurobiological model (visually induced disgust signature, VIDS) for subjective disgust experience. Specifically, the developed VIDS accurately predicted momentary self-reported subjective disgust across discovery and validation cohorts, both on the population-level and individual-level. Moreover, the VIDS robustly generalized across samples, study contexts and MRI systems with regard to accurately capturing disgust experience in response to core disgust stimuli (n=34 and n=26), oral distaste (n=30) and unfair offers in an ultimatum game task (socio-moral disgust; n=43), suggesting that the experience of disgust may extend from the gustatory over the subjective experience and into the socio-moral domain (see also evolutionary models proposed in ref.2).
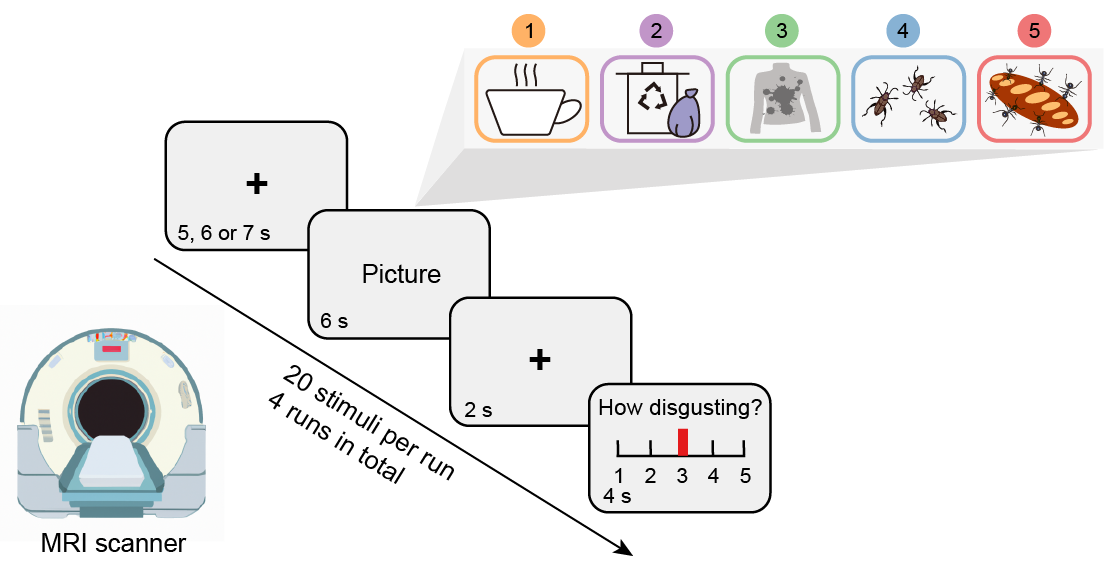
Figure 1. fMRI disgust induction paradigm. Of note, schematic figures were used for display purpose and were not included in the original stimulus set.
Subjective disgust is associated with and predicted by distributed neural systems
Previous studies emphasized the contribution of specific brain regions, such as the insula4,5, amygdala4, salience network6 and default mode network (DMN)7, to subjective disgust experience. Through a series of systematical analyses, we found that capturing disgust experience requires concerted engagement of brain-wide distributed cortical and subcortical systems, while no isolated region or network reached the predictive accuracy of the whole-brain model (i.e., VIDS).
The experience of disgust, fear and non-specific negative affect have shared yet distinguishable neurofunctional representations
In a series of analyses, we further demonstrated that subjective disgust experience showed shared yet separable neural representations with established predictive models for the subjective experience of non-specific negative affect (picture induced negative affect signature, PINES8) or fear (visually induced fear signature, VIFS9), such that all models engaged subcortical regions and regions implicated in emotional awareness and appraisal, whereas the neural signature of disgust robustly mediated the response of the other two models (PINES and VIFS) on subjective disgust ratings but not vice versa. Although the three neural signatures showed a certain extent of similarity in the range of intense emotional experiences (for a similar observation, see also ref.10), the signatures most accurately predicted the respective target experience.
Why is this study important?
We provide an accurate fMRI signature for disgust with a high potential to resolve ongoing evolutionary debates (Figure 2). This study adds to the understanding of the neurobiological underpinnings of subjective disgust experience. Disgust dysregulations play a role in a number of mental disorders11-13, and the resulting subjective disgust marker can be potentially useful to examine novel treatments (for initial applications of MVPA-based decoders in treatment and intervention evaluation, see refs.14,15).

Figure 2. Disgust evaluation model
References
- Rozin, P., Haidt, J., and Fincher, K. (2009). From oral to moral. Science 323, 1179-1180. https://doi.org/10.1126/science.1170492.
- Chapman, H.A., and Anderson, A.K. (2013). Things rank and gross in nature: a review and synthesis of moral disgust. Psychol. Bull. 139, 300-327. https://doi.org/10.1037/a0030964.
- Chapman, H.A., Kim, D.A., Susskind, J.M., and Anderson, A.K. (2009). In bad taste: evidence for the oral origins of moral disgust. Science 323, 1222-1226. https://doi.org/10.1126/science.1165565.
- Gan, X., Zhou, X., Li, J., Jiao, G., Jiang, X., Biswal, B., Yao, S., Klugah-Brown, B., and Becker, B. (2022). Common and distinct neurofunctional representations of core and social disgust in the brain: coordinate-based and network meta-analyses. Neurosci. Biobehav. Rev. 135, 104553. https://doi.org/10.1016/j.neubiorev.2022.104553.
- Wicker, B., Keysers, C., Plailly, J., Royet, J.-P., Gallese, V., and Rizzolatti, G. (2003). Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron 40, 655-664. https://doi.org/10.1016/S0896-6273(03)00679-2.
- Pujol, J., Blanco-Hinojo, L., Coronas, R., Esteba-Castillo, S., Rigla, M., Martínez-Vilavella, G., Deus, J., Novell, R., and Caixàs, A. (2018). Mapping the sequence of brain events in response to disgusting food. Hum. Brain Mapp. 39, 369-380. https://doi.org/10.1002/hbm.23848.
- Satpute, A.B., and Lindquist, K.A. (2019). The default mode network’s role in discrete emotion. Trends Cogn. Sci. 23, 851-864. https://doi.org/10.1016/j.tics.2019.07.003.
- Chang, L.J., Gianaros, P.J., Manuck, S.B., Krishnan, A., and Wager, T.D. (2015). A sensitive and specific neural signature for picture-induced negative affect. PLoS Biol. 13, e1002180. https://doi.org/10.1371/journal.pbio.1002180.
- Zhou, F., Zhao, W., Qi, Z., Geng, Y., Yao, S., Kendrick, K.M., Wager, T.D., and Becker, B. (2021). A distributed fMRI-based signature for the subjective experience of fear. Nat. Commun. 12, 6643. https://doi.org/10.1038/s41467-021-26977-3.
- Wager, T.D., Krishnan, A., and Hitchcock, E. (2018). How are emotions organized in the brain? In The nature of emotion. Fundamental questions, A.S. Fox, R.C. Lapate, A.J. Shackman, and R.J. Davidson, eds. (Oxford University Press), pp. 112-118.
- Vicario, C.M., Rafal, R.D., Martino, D., and Avenanti, A. (2017). Core, social and moral disgust are bounded: a review on behavioral and neural bases of repugnance in clinical disorders. Neurosci. Biobehav. Rev. 80, 185-200. https://doi.org/10.1016/j.neubiorev.2017.05.008.
- Amoroso, C.R., Hanna, E.K., LaBar, K.S., Schaich Borg, J., Sinnott-Armstrong, W., and Zucker, N.L. (2019). Disgust theory through the lens of psychiatric medicine. Clin. Psychol. Sci. 8, 3-24. https://doi.org/10.1177/2167702619863769.
- Bhikram, T., Abi-Jaoude, E., and Sandor, P. (2017). OCD: obsessive–compulsive … disgust? The role of disgust in obsessive–compulsive disorder. J. Psychiatry Neurosci. 42, 300. https://doi.org/10.1503/jpn.160079.
- Reddan, M.C., Wager, T.D., and Schiller, D. (2018). Attenuating neural threat expression with imagination. Neuron 100, 994-1005.e1004. https://doi.org/10.1016/j.neuron.2018.10.047.
- Zhang, R., Zhao, W., Qi, Z., Xu, T., Zhou, F., and Becker, B. (2023). Angiotensin II regulates the neural expression of subjective fear in humans: a precision pharmaco-neuroimaging approach. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 8, 262-270. https://doi.org/10.1016/j.bpsc.2022.09.008.
Follow the Topic
-
Nature Human Behaviour

Drawing from a broad spectrum of social, biological, health, and physical science disciplines, this journal publishes research of outstanding significance into any aspect of individual or collective human behaviour.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in