Safety of COVID-19 vaccination in children
Published in Paediatrics, Reproductive Medicine & Geriatrics
The COVID-19 pandemic has affected millions of children globally with major health, social and educational consequences. Vaccines are one of the first lines of defence against infectious diseases and were crucial for curbing the COVID-19 pandemic and allowing us to go back to normal life. Findings from large-scale clinical trials showed that vaccination was safe and effective against serious outcomes from COVID-19 infection (e.g. hospitalisation) in both adults and children. However, the benefits of COVID-19 vaccination were less clear cut in children compared to adults, as children were less likely than adults to become seriously ill from COVID-19 infection.
Concerns around vaccine safety are a barrier to vaccine acceptance generally, and this was particularly highlighted during the COVID-19 pandemic due to the rapid development and rollout of new vaccines. For example, the association of myocarditis with mRNA COVID-19 vaccines (particularly in young adult males) was especially concerning. As such, it is important for parents and young people to have access to reliable information about the safety of these vaccines to help them make informed decisions regarding vaccination. Therefore, we conducted a large-scale analysis of COVID-19 vaccine safety in children in England using routinely collected data, in a study funded by the National Institute for Health and Care Research (NIHR).
What did we do?
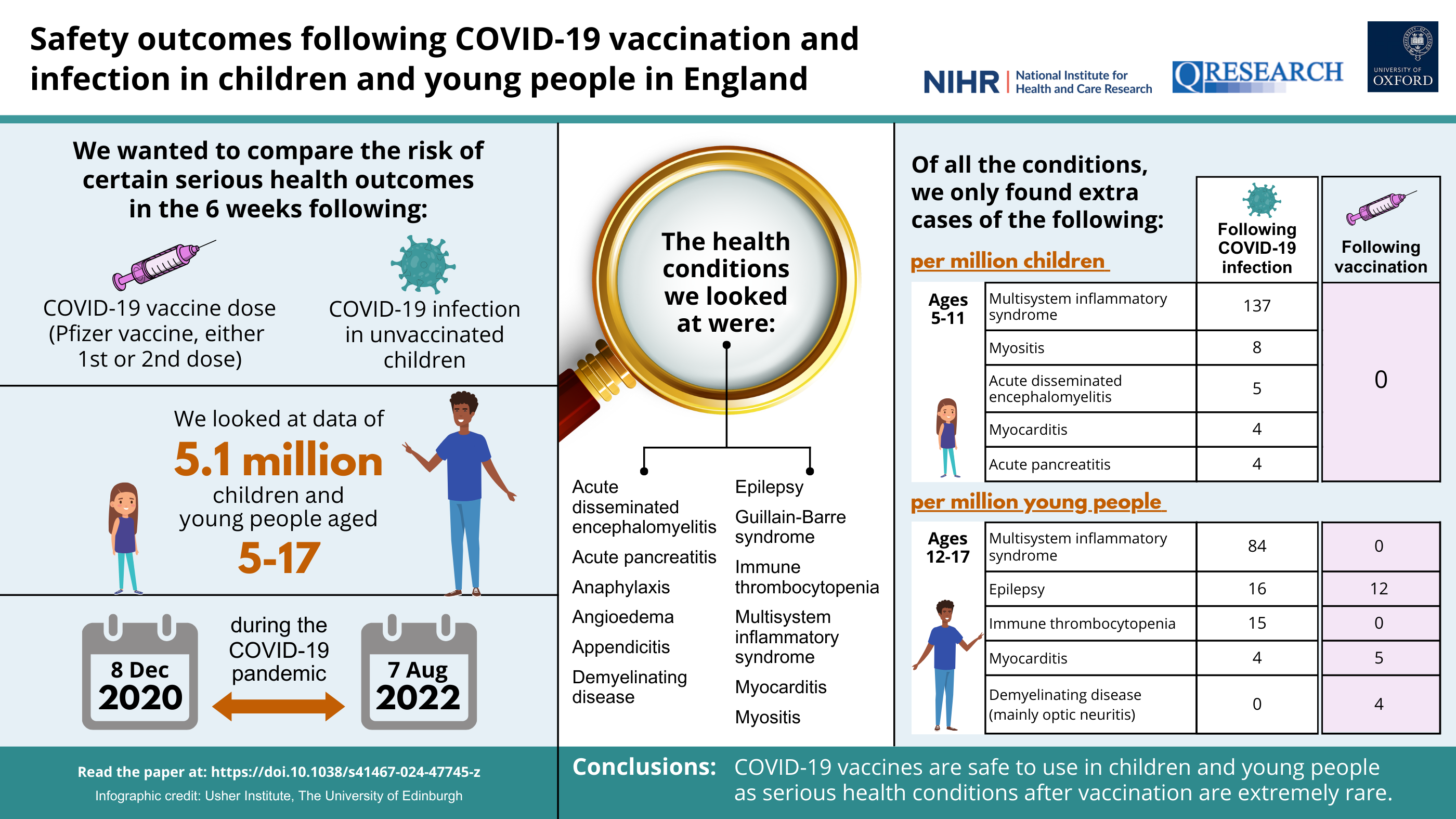
We looked at data from over 5.1 million children and young people aged 5-17 years in England using the National Immunisation Database, linked to the QResearch primary care database (GP records), hospital records and COVID-19 PCR testing data. Based on the scientific literature and clinical expertise, we selected 12 outcomes that are potentially associated with COVID-19 vaccination, infection, or vaccination more generally, to look at in our study. These outcomes were:
- Multi-system inflammatory syndrome (inflammation throughout the body caused by COVID-19 infection)
- Myocarditis (inflammation of the heart)
- Myositis (inflammation of the muscles causing muscle weakness)
- Immune thrombocytopenia (bruising and bleeding due to low platelet levels in the blood)
- Epilepsy (condition that affects the brain and is characterised by frequent seizures)
- Appendicitis (inflammation of the appendix)
- Acute disseminated encephalomyelitis (inflammatory condition that affects the brain and spinal cord following infection)
- Acute pancreatitis (inflammation of the pancreas)
- Angioedema (swelling of the deeper layers of the skin)
- Demyelinating disease (group of conditions linked to inflammation of the brain or spinal cord)
- Guillain-Barre Syndrome (autoimmune disorder that affects the nerves)
- Anaphylaxis (severe allergic reaction)
We looked at whether there was an increased risk of any of these outcomes in the 6 weeks following a first, second or third vaccine dose between December 2020 and August 2022. We looked at the main types of COVID-19 vaccine available at the time, which included Pfizer-BioNTech and Moderna (also known as Spikevax), which are mRNA vaccines, and the Oxford-AstraZeneca vaccine, which is a viral vector vaccine. The Oxford-AstraZeneca vaccine was only given to a small number of under-18s as it was not approved for use in people under the age of 40 in the UK from April 2021.
COVID-19 vaccine safety in young people aged 12-17 years
COVID-19 vaccination was offered to all young people aged 12 and above in September 2021. By August 2022, over 2.8 million 12-17-year-olds included in our study had received a first dose of COVID-19 vaccine, over 2.2 million had received a second dose and over 400,000 had received a third dose. Over 99% of first and second doses in 12-17-year-olds used the Pfizer-BioNTech vaccine. The Pfizer-BioNTech vaccine was also predominantly used for the third booster dose in this age group with some receiving Moderna instead.
In young people aged 12-17, we found a small increased risk of myocarditis in the 6 weeks following a first or second dose of the Pfizer-BioNTech vaccine. We estimated that for every 1 million young people vaccinated with a first dose of Pfizer-BioNTech, there would be an additional 3 cases of myocarditis, and an additional 5 cases following a second dose. However, we did not find an increased risk of myocarditis following vaccination with the Moderna vaccine in adolescents, as some previous studies have reported.
We also found a small increased risk of hospitalisation with epilepsy in 12-17-year-olds following a second dose of the Pfizer-BioNTech vaccine (12 extra cases per million vaccinated). However, this is likely to reflect the exacerbation of seizures in children who already have epilepsy rather than new cases of epilepsy being triggered by the vaccine, as seizure exacerbation in adults with epilepsy following COVID-19 vaccination has been reported in previous studies. Additionally, a new diagnosis of epilepsy would usually take longer than the 6-week period that we looked at in this study.
In girls aged 12-17, we found an increased risk of 'demyelinating disease' following a second dose of the Pfizer-BioNTech vaccine that was not observed in boys (4 cases per million vaccinated). The majority of these cases were inflammation of the optic nerve, which is a condition that affects females more than males and is generally associated with good visual recovery in most childhood cases. To date, there has only been weak evidence linking mRNA vaccines to more serious demyelinating conditions.
While the Oxford-AstraZeneca vaccine was only given to a small number of young people in the UK (less than 1% in our study), we identified notable increased risks of hospitalisation with epilepsy (705 extra cases per million vaccinated) and appendicitis (512 extra cases per million vaccinated) following a first or second dose of Oxford-AstraZeneca vaccine. Our results suggest that further work would need to be done to ensure the safety of this vaccine in young people if it were to be used in future vaccination programmes.
COVID-19 vaccine safety in children aged 5-11 years
In April 2022, the UK's COVID-19 vaccination programme was extended to all children aged 5 and above. Our study included over 1.8 million children in this age group. During the study period, nearly 600,000 of these children had received a first dose of COVID-19 vaccine, and over 300,000 had received a second dose. Over 99% of these vaccine doses were the Pfizer-BioNTech vaccine. Reassuringly, we found no increased risks of any of the aforementioned outcomes in the 6 weeks following a first, second or third dose of Pfizer-BioNTech, Moderna or Oxford-AstraZeneca COVID-19 vaccines.
Conclusion
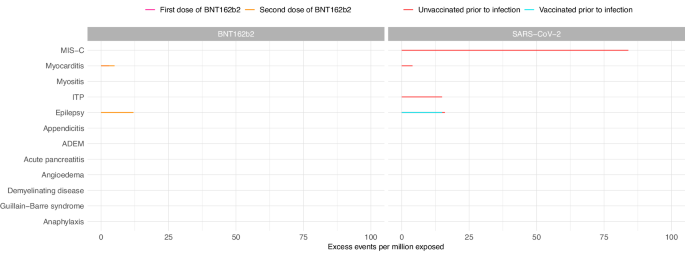
The risks of these serious health outcomes following COVID-19 infection in children and young people clearly outweigh the risks following COVID-19 vaccination (as shown in Fig. 1). When further considering the risk of serious illness caused by COVID-19 itself, and the proven effectiveness of COVID-19 vaccines in protecting against these serious outcomes in children, our study provides robust evidence that COVID-19 vaccination (with the Pfizer-BioNTech vaccine) is safe for use in children and young people.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in