Schizophrenia related protein SAP97 displays spontaneous nucleation and Ca2+ dependent variability in real-time dynamics.
Published in Healthcare & Nursing

A journey to the mysterious signalling islands
Over the last decade, synaptopathy is implicated in the onset of several neuropsychiatric disorders1 2 3. Our understanding has evolved over years to comprehend the heterogeneity of protein expression, trafficking and local organization within individual synapses that contribute to progression of several diseases. Recent evidences converge to support a direct connection between the loss of function of synapse associated protein-97 (SAP97), a member of the membrane-associated guanylate kinase (MAGUK) family of scaffolding proteins, and psychiatric disorders like schizophrenia and autism spectrum disorders4 5 6 7. At excitatory postsynapse, the molecular meshwork smaller than the size of individual synapses known as “nanodomains” or “nanocolumns”8 9 10 11 , traps the molecules involved in neurological as well as neurodegenerative disorders. Notably, these nanoscale signalling islands place the glutamate receptors adjacent to the presynaptic release site, controlled by PSD95, PSD93, SAP102 and SAP97, the four prominent members of MAGUKs 12 13. Till date SAP97 is the only one among them to directly associate with the activity dependant subunit GluA1 of AMPA type glutamate receptors in post synaptic density (PSD)14 15.
Molecular behaviour that aids the formation, stabilisation, and recycling of nanoscale domains in synapses is getting re-evaluated vigorously in vitro and in vivo 9 10 16 17. The components that form these reversible functional domains can aggregate either homotypically or heterotypically to optimise the efficacy of information transfer. A growing body of evidence confirms the existence of regulatory nanodomains in neurons and non-neuronal cells, which are formed transiently in real-time at spatial scales of 10-200 nm in cells11 18 19. Last few years have provided tremendous insights using in vitro observations on how some of these molecules can separate into micron scale condensates20 21 . In case of synaptic molecules, or molecules involved in modulating and regulating the translation, they can spontaneously nucleate into condensates with distinct kinetics of phase separation and local compositionality. These studies highlight that liquid-liquid phase separation (LLPS) can effectively steer the formation of transient domains which serve as a nucleating architecture at molecular resolution. Using SAP97 as a model we evaluated the micron scale in vitro protein condensates and their in vivo nano condensates in both heterologous cell lines and primary hippocampal neurons, along with the role of isoform composition in nucleation of these condensates.
We performed direct stochastic optical reconstruction microscopy (dSTORM), a super-resolution technique, to understand the endogenous distribution of SAP97 in heterologous cells22 23. We observed that nanoscale segregation of SAP97 was physiologically regulated by the availability of free Ca2+ and modulated through the Ca2+ sensing protein Calmodulin (CaM). Using single-molecule localisation microscopy in combination with Ca2+ perturbation and analysis paradigms to evaluate the free energy change (ΔG) in protein aggregation, we show that the nanoscale condensation of SAP97 followed a first-order phase transition with spontaneous nucleation and growth. These nano condensates displayed an altered phase transition in response to differential modulation of intracellular Ca2+ levels. Since SAP97 can associate with Ca2+ bound CaM, we confirmed the presence of its C-terminal spliced isoforms in the hippocampal and cortical regions of rodent brains as well as in heterologous cell lines. These isoforms are known to alter the dynamics of several cell surface molecules, including cell adhesion molecules, channels, and receptors such as AMPA receptors, which are known to form distinct subsynaptic nanodomains8 9 10 11.
Additionally, using Fluorescence Recovery after Photobleaching (FRAP) in combination with Total Internal Reflection Fluorescence (TIRF) illumination in live neuroblastoma cells, we evaluated the exchange of SAP97 isoforms in a diffraction-limited compartment. The rate of exchange of alternatively spliced C-terminal variants of SAP97 was distinct from each other. This local exchange of each isoform was influenced by cytoplasmic Ca2+ bound CaM levels and was modulated by the interaction of the intrinsically disordered HOOK region of SAP97. Furthermore, in vitro phase transition studies confirmed that the isoforms of SAP97 can transition into phase-separated condensates and co-condense Calmodulin in a Ca2+-dependent manner. We confirmed that these differential effects of isoform expression at the micron scale are also conserved in vivo at the nanoscale using dissociated hippocampal neurons and neuroblastoma cells. The local composition or the ratio between different isoforms yielded comparable molecular signatures of nucleation of phase separated condensates. Additionally, in matured neurons the condensates of SAP97 were observed to be localised at the periphery of the PSD as previously reported24, where the combination of isoforms could modulate the properties of the condensates and further regulate the plasticity mechanisms involved at individual synapses.
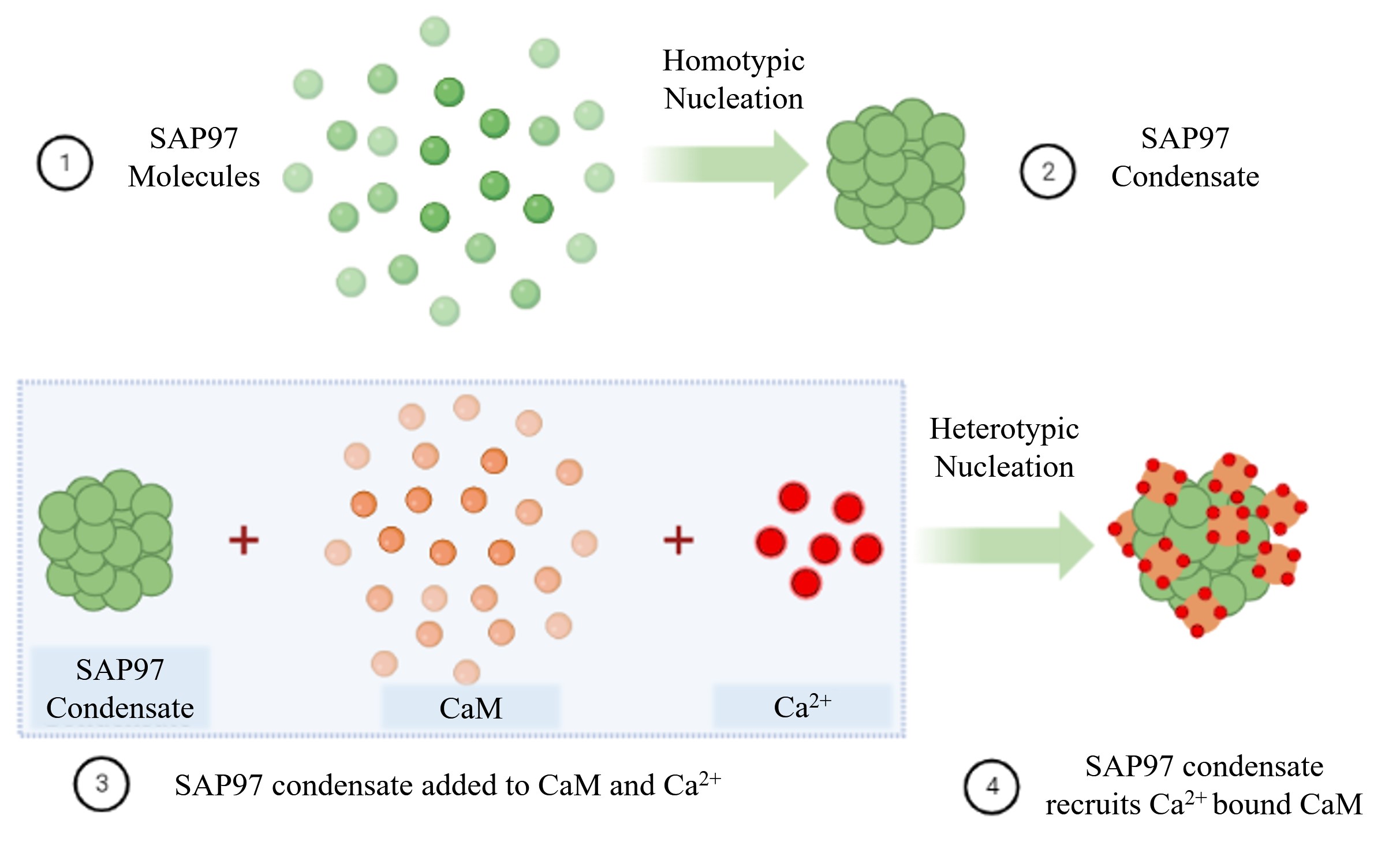
This study from our lab explores the intricacies involved in the phase separation of molecules and complex signalling mechanisms at nano-micron scales in cellular systems and in solution. The observation of SAP97 nano condensates can contribute to regulation of reserve pool of glutamatergic receptor islands in proximity to the PSD and influence an activity dependent molecular tuning through local availability of Calcium and Calcium binding proteins. Understanding the interplay of these biomolecules and their complexities at nanoscale will aid us in understanding how self-organization contribute to heterogeneity in synaptic responses resulting in altered cognition in health and disease.
References
- Bayés, A. et al. Characterization of the proteome, diseases and evolution of the human postsynaptic density. Nat. Neurosci. 14, 19–21 (2011).
- De Rubeis, S. et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515, 209–215 (2014).
- Luo, J., Norris, R. H., Gordon, S. L. & Nithianantharajah, J. Neurodevelopmental synaptopathies: Insights from behaviour in rodent models of synapse gene mutations. Prog. Neuropsychopharmacol. Biol. Psychiatry 84, 424–439 (2018).
- Uezato, A. et al. Genetic and molecular risk factors within the newly identified primate‐specific exon of the SAP97/DLG1 gene in the 3q29 schizophrenia‐associated locus. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 174, 798–807 (2017).
- Fromer, M. et al. De novo mutations in schizophrenia implicate synaptic networks. Nature 506, 179–184 (2014).
- Xing, J. et al. Resequencing and Association Analysis of Six PSD-95-Related Genes as Possible Susceptibility Genes for Schizophrenia and Autism Spectrum Disorders. Sci. Rep. 6, 27491 (2016).
- Marshall, C. R. et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat. Genet. 49, 27–35 (2017).
- Nair, D. et al. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J. Neurosci. (2013). doi:10.1523/JNEUROSCI.2381-12.2013
- Tang, A.-H. et al. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature 536, 210–214 (2016).
- Biederer, T., Kaeser, P. S. & Blanpied, T. A. Transcellular Nanoalignment of Synaptic Function. Neuron 96, 680–696 (2017).
- Choquet, D., Sainlos, M. & Sibarita, J.-B. Advanced imaging and labelling methods to decipher brain cell organization and function. Nat. Rev. Neurosci. 22, 237–255 (2021).
- Won, S., Levy, J. M., Nicoll, R. A. & Roche, K. W. MAGUKs: multifaceted synaptic organizers. Curr. Opin. Neurobiol. 43, 94–101 (2017).
- Kim, E. & Sheng, M. PDZ domain proteins of synapses. Nat Rev Neurosci 5, 771–781 (2004).
- Fourie, C., Li, D. & Montgomery, J. M. The anchoring protein SAP97 influences the trafficking and localisation of multiple membrane channels. Biochim. Biophys. Acta - Biomembr. 1838, 589–594 (2014).
- Waites, C. L. et al. Synaptic SAP97 Isoforms Regulate AMPA Receptor Dynamics and Access to Presynaptic Glutamate. J. Neurosci. 29, 4332–4345 (2009).
- Zeng, M. et al. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell 166, 1163-1175.e12 (2016).
- Zeng, M. et al. Reconstituted Postsynaptic Density as a Molecular Platform for Understanding Synapse Formation and Plasticity. Cell 174, 1172-1187.e16 (2018).
- Padmanabhan, P., Kneynsberg, A. & Götz, J. Super-resolution microscopy: a closer look at synaptic dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 22, 723–740 (2021).
- Venkatesan, S. et al. Differential scaling of synaptic molecules within functional zones of an excitatory synapse during homeostatic plasticity. eNeuro (2020). doi:10.1523/ENEURO.0407-19.2020
- Narayanan, A. et al. A first order phase transition mechanism underlies protein aggregation in mammalian cells. Elife (2019). doi:10.7554/eLife.39695
- Poudyal, M. et al. Liquid condensate is a common state of proteins and polypeptides at the regime of high intermolecular interactions. (2022). doi:10.1101/2021.12.31.474648
- Heilemann, M. et al. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. Engl. 47, 6172–6176 (2008).
- van de Linde, S. et al. Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nat. Protoc. 6, 991–1009 (2011).
- DeGiorgis, J. A., Galbraith, J. A., Dosemeci, A., Chen, X. & Reese, T. S. Distribution of the scaffolding proteins PSD-95, PSD-93, and SAP97 in isolated PSDs. Brain Cell Biol. 35, 239–250 (2006).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in