Single-Transistor Neuron with Excitatory-Inhibitory Spatiotemporal Dynamics Applied for Neuronal Oscillations
Published in Electrical & Electronic Engineering

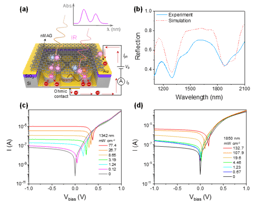
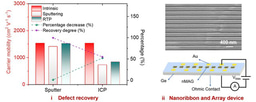
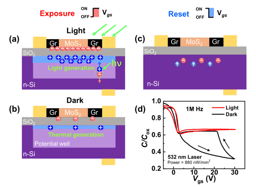
This work demonstrated a single-transistor artificial excitatory-inhibitory (E-I) neuron by integrating a threshold switch connected with a gated 2D semiconductor MoS2 channel. Without auxiliary reset circuits, the device exhibits bio-realistic dynamics to emulate essential neuronal functions such as leaky integration, threshold-driven fire, inhibition, firing threshold tuning, and self-recovery. The biomimetic neuron operates with independently regulated excitatory and inhibitory inputs. These unique designs allow the spatiotemporal integration of excitatory and inhibitory signals to be realized in a single device without any circuits.
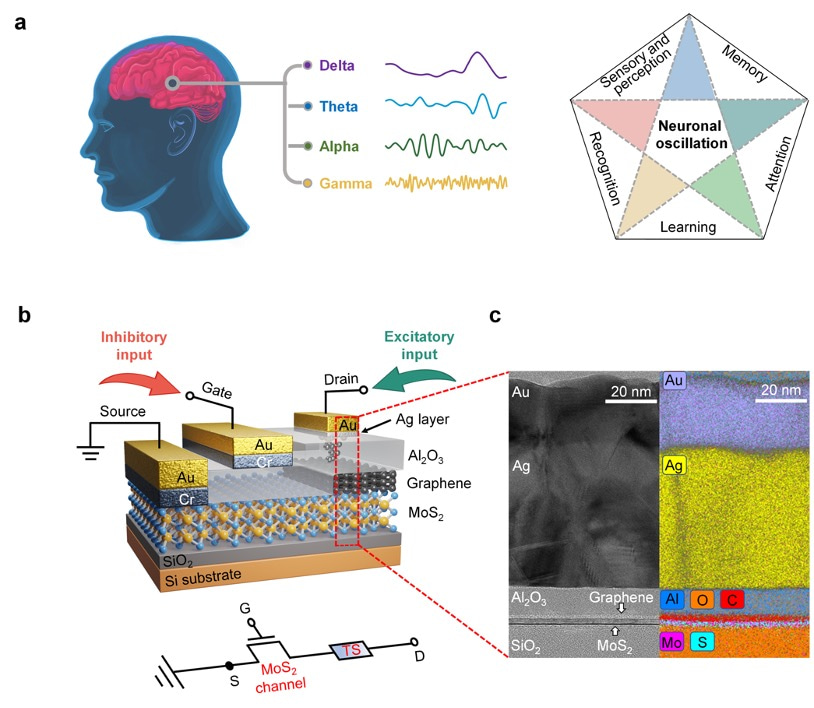
More importantly, the artificial E-I neuron can be adopted to generate neuronal oscillations and support information integration and synchronization. Neuronal oscillations originated from the synergistic effect of E-I signals, and constructs high-dimensional population codes to enable precise shuffling/integration of bottom-up and top-down signals. The E-I neuron demonstrates advanced neuronal functions and provides a promising pathway to building ultra-scalable, low-cost, basic components for large-scale integrated neuromorphic systems with superior brain-inspired functions.
Brain-inspired neuromorphic systems with highly parallel processing capabilities, scalability, and low-power consumption offer a promising solution to the von Neumann bottleneck, providing a key route toward artificial general intelligence. In the brain’s hierarchical structure and neuro-synaptic framework, the neuron behaves as the basic unit of information processing. Neurons and synapses are responsible for different roles. Neurons perform stimuli integration and process, exhibiting threshold-driven firing response and leaky integration. Synapses modulate the information flow through tuning their weights. Significant progress has been made on artificial synapses, while biomimetic neuron is less reported. Memristors based on the diffusion dynamics of Ag have been proposed to act as neuromorphic devices. Diffusive Ag-in-oxide memristors’ nonvolatile dynamics enable a direct emulation of synaptic plasticity, while Ag-based memristors with volatile switching effect can perform neuron’s functions. The next level is neuronal circuits, which have specialized excitatory–inhibitory (E–I) connection patterns to realize diverse functions and build complex architectural plans. Core neuronal circuits represent basic building blocks of cortical architecture and spatially combine functions of excitation and inhibition. In the biological systems, the primary means by which stimuli is delivered from one neural region to another is through excitatory signals, while inhibitory signals modulate the information flow locally and contribute to sparse-activity-induced low power operations. To make key advances in neuromorphic systems, a core research focus is to implement biomimetic and ultracompact components with the potential to construct neuronal circuits with various scales. Therefore, artificial neuron with E–I spatiotemporal dynamics is highly desired. Although artificial neurons with different designs had been developed, most lack the inhibitory function despite its equal importance with excitatory function. To address the issue, researchers use complex circuits to build E–I neurons in hardware. The E–I neurons comprise multiple capacitors and resistors plus additional circuits or have a common input for excitatory and inhibitory signals. Unfortunately, these schemes suffer from escalated hardware cost and power budget, missing functions, and poor scalability; they also impede the enactment of spatiotemporal dynamics that simultaneously process the excitatory and inhibitory signals. Neuromorphic networks usually adopt the Poisson code to encode information into spike trains where neurons need to fire a huge excess of spikes to reach an acceptable precision, which is limited by the unavoidable discretization. However, there is increasing evidence that the biological networks with a balance between excitatory and inhibitory inputs may provide efficient and cooperative code at the population level, thus significantly improving the network’s performance. As a significant mode of brain communication, neuronal oscillations can construct high-dimensional population codes through neuron clusters’ synchronization of action potentials and are widely implicated in brain activities. For example, neuronal oscillations with gamma rhythm are considered to denote the reference signals for temporal encoding, promoting information exchange. The critical functions of neuronal oscillations include perception,[19] learning, and memory, and others. Neuronal oscillations are considered to coordinate brain activity into ordered patterns that optimize local information processing and facilitate signal transmission, constituting a fundamental mechanism to enable precise shuffling and integration of bottom-up and top-down signals. Thus, it is vital to replicate the neuronal oscillations with artificial hardware as a key effort in neuromorphic computing. We demonstrate a single-transistor artificial E–I neuron by integrating a threshold switch (TS) connected with a gated 2D semiconductor MoS2 channel. Without auxiliary reset circuits, the device exhibits bio-realistic dynamics to emulate essential neuronal functions such as leaky integration, threshold-driven fire, inhibition, firing threshold tuning, and self-recovery. The biomimetic neuron operates with independently regulated excitatory and inhibitory inputs. These unique designs allow the spatiotemporal integration of excitatory and inhibitory signals to be realized in a single device without any circuits. More importantly, the artificial E–I neuron can be adopted to generate neuronal oscillations and support information integration and synchronization. As a result, the E–I neuron demonstrates enriched functions and provides a promising pathway to building ultrascalable, low-cost, basic components for largescale integrated neuromorphic systems with superior braininspired functions.
Neuronal oscillations are essential activities associated with advanced brain functions. The network of fast-spiking and soma-inhibiting interneurons plays a crucial role in generating neuronal oscillations with gamma rhythm. As soma-inhibiting GABA-containing interneurons (GABA is an inhibitory neurotransmitter), basket cells’ action potentials are generated at a rate of ≈1 per gamma cycle and are precisely phase-locked to the oscillations. Therefore, regular and synchronized neuronal activity is critical for generating oscillations. In the inhibitory interneuron networks, the fast and robust inhibition signal among interneurons is an effective synchronization. In vivo analysis reveals that the oscillation generation also requires the excitatory synapses between pyramidal cells and intermediate neurons with excitatory neurotransmitters such as AMPA. The compound inhibitory conductance formed by the inhibitory and excitatory signals underlies the synchronization in interneuron networks, which would further generate neuronal oscillations. The temporal windows of high (red) and low excitability (blue) defined by the compound inhibitory conductance follow an alternating manner with the capability of regulating the neurons’ activity in the oscillating interneuron network (taking five neurons as an example). We established a pyramidal-interneuron gamma (PING) network to reveal and characterize the generation of neuronal oscillations with gamma rhythm. The excitatory pyramidal cells (E-cells) and the inhibitory interneurons (I-cells) are closely distributed. The activation of the reciprocally connected networks of excitatory and inhibitory neurons via the PING mechanism is considered one of the significant ways to produce synchrony in a minicolumn (details of the PING network simulation can be found in Text S1, Supporting Information). Figure 5e plots the normalized density of firing events of I-cells varying with time (estimated using a Gaussian kernel with a bandwidth of 3 ms). At the beginning of the simulation, neurons fire irregularly, while under the modulation of E-cells’ activation and I-cells’ suppression, neurons’ firing is gradually synchronized to generate gamma oscillation. We further study the impacts of the I-cells’ modulation strength on the synchronization The PING network simulation indicates that a vital sign of neuronal oscillation generation is the normalized density of interneurons firing events changing periodically with the specific rhythm.
https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202207371

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in