Soft Materials From Thin Air
Polymers from abundant CO2
As the world is committed to reduce its carbon dioxide (CO2)—one of the primary greenhouse gases—emissions globally, chemists wonder how to create carbon-negative processes to actively incorporate the greenhouse gas into value-added materials. Carbon capture and storage (CCS) technology aims to reduce atmospheric CO2 concentrations, but a critical question remains: beyond storage, how can captured CO2 be sustainably utilized? Surely after satisfying the global demand for carbonated drinks there is still a significant amount unaccounted for.
One promising use of captured CO2 is to make polymers, creating valuable materials from this common gas. Aliphatic polycarbonates, made by combining CO2 with epoxide monomers, have already shown promise in making flexible films and coatings. Scientists have also tried to combine CO2 directly with ethylene — a fossil-derived feedstock chemical — to create eco-friendly plastics similar to polyethylene. Unfortunately, this has proven difficult because of unfavorable thermodynamics, with the result that ethylene polymerizes by itself without incorporating CO2.
However, the reaction of CO2 with butadiene, another hydrocarbon consisting of two ethylene units connected by a C-C bond, has been realized, affording a six-membered lactone, containing 1x CO2 and 2x butadiene dubbed “EVP”. This molecule has been known for several decades but its potential to create sustainable materials were overlooked for a long time. In 2014, the Nozaki group described the polymerization of EVP, which led to a wave of publications on new polymerization methods of this monomer and its derivatives into attractive soft materials. In parallel the synthesis of this molecule has been continuously improved.
Plastics from two climate-warming gases
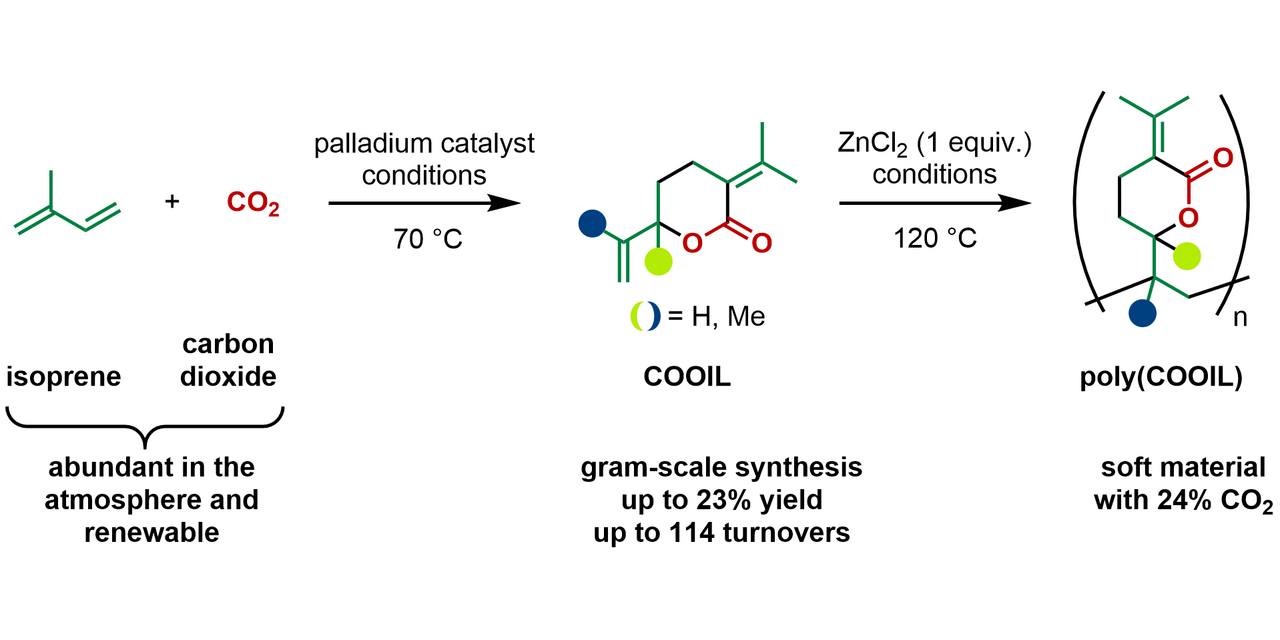
Ever since the discovery of poly(EVP), we were wondering if we could replace butadiene with its homolog isoprene that contains an additional methyl group. Now nearly 10 years after our initial discovery, we can report the telomerization of CO2 with isoprene.
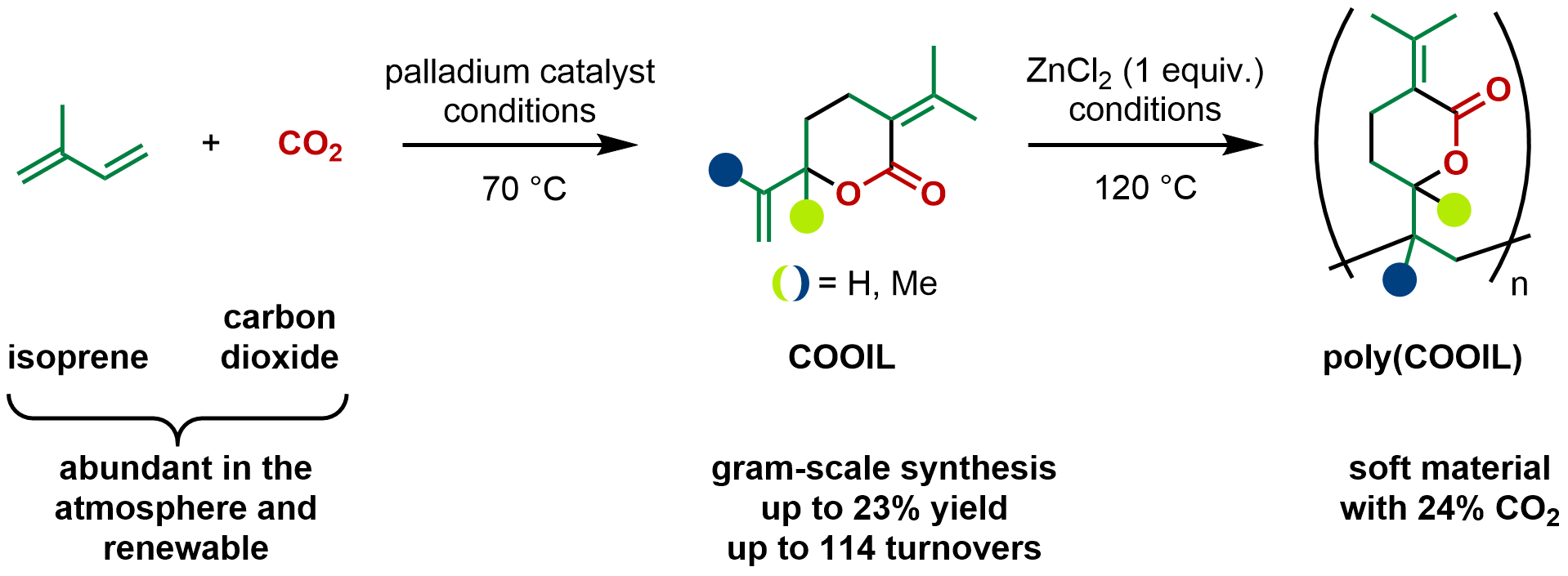
A look in the literature revealed that already in the 1980’s chemists tried this reaction but only managed to isolate miniscule amounts of the corresponding lactone. It is believed that the additional methyl groups cause steric congestion and disfavor some of the catalytic steps. We were intrigued by the chemical challenge and when we looked deeper at the role of isoprene on our planet, we were astonished to learn that isoprene is emitted on a massive scale by forests (estimated at around 500 million tonnes per year), matching global emissions of methane, a well-known climate gas and energy resource. We reasoned that turning the abundant CO2 and isoprene into an easy-to-handle and densely functionalized lactone could be an attractive reaction for organic chemists. Moreover, it could serve as an attractive starting point to kick off a second wave of material applications based on this potential monomer related to EVP.
When we ran the first experiments in the lab, we were hit with setbacks – low conversion and only side products without CO2 incorporation. Tirelessly we kept investigating the reaction parameters, drawing from the learnings made with the butadiene analog. Then we found that tetrabutylammonium acetate, or TBAAc in short, promoted high selectivity towards the desired lactone product, although the yield remained still in the low single digits. Gradually we were able to increase the yield by fine tuning the reaction parameters and finally found that the combination of a palladium (II) salt, a phosphine ligand, TBAAc and acetonitrile as solvent afforded the highest selectivity and conversion of isoprene. Everything was progressing smoothly, until the day we ran out of the bottle of said TBAAc. With the new batch the reaction returned to its initial unselective state, causing us a headache until we isolated trace amounts of water as the root cause. By precisely controlling the amount of water we succeeded in producing the CO2-isoprene lactone (“COOIL”) reliably and on gram scale, a breakthrough.

After concluding the experimental work, we were interested in understanding the mechanism of the reaction and turned to DFT calculations to answer questions regarding the reaction pathway and the selectivity; in fact we only isolated two out of four possible lactone isomers we drew up on paper. This is different from butadiene, because the “head” and the “tail” of two isoprene units can match up to form distinct dimers. According to our calculations, the dimerization of two isoprene units is fast and can occur in all orientations, but is also reversible. The insertion of CO2 then takes place to form a new C-C bond and a palladium carboxylate species. The turnover-limiting step is the cyclization that forms the C-O bond. In the transformation we formed two isomers, one being the head-to-tail and the other the tail-to-tail dimer. The latter is lower in energy while the former benefits from a lower barrier for the reductive elimination. We also found that two more potential products are not formed because of too high barriers.
From monomer to polymer
With sufficient amounts of COOIL monomer in hand, we moved to polymerize it, a task that proved more challenging than expected, often resulting in monomer recovery rather than polymer formation. Drawing inspiration from our previous work, we could polymerize COOIL under the action of zinc chloride and study some of the physical properties of poly-COOIL. Preliminary studies revealed that poly-COOIL has a lower glass transition temperature compared to poly-EVP, suggesting potential applications in films and coatings.
We hope this research inspires further innovation in improving monomer synthesis and exploring the practical uses of polymers derived from CO₂ and isoprene—two abundant organic molecules in the atmosphere.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Ask the Editor – Polymers
Got a question for the editor about Functional polymers? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in