Glycosides play a diverse array of roles in living organisms, acting not only as sources of energy and structural components but also as vital mediators of numerous physiological processes. Among glycosides, carbon-glycosides demonstrate superior metabolic stability compared to their oxygen- and nitrogen-based counterparts. As a result, carbon glycosides have garnered considerable attention in the realm of drug discovery and development. Additionally, quaternary glycosides hold significant value and form the foundation of several pharmaceutical compounds. Nevertheless, the stereoselective construction of quaternary centers on sugar substrates remains a complex challenge, driving researchers to develop alternative methodologies for the synthesis of complex glycoside structures.
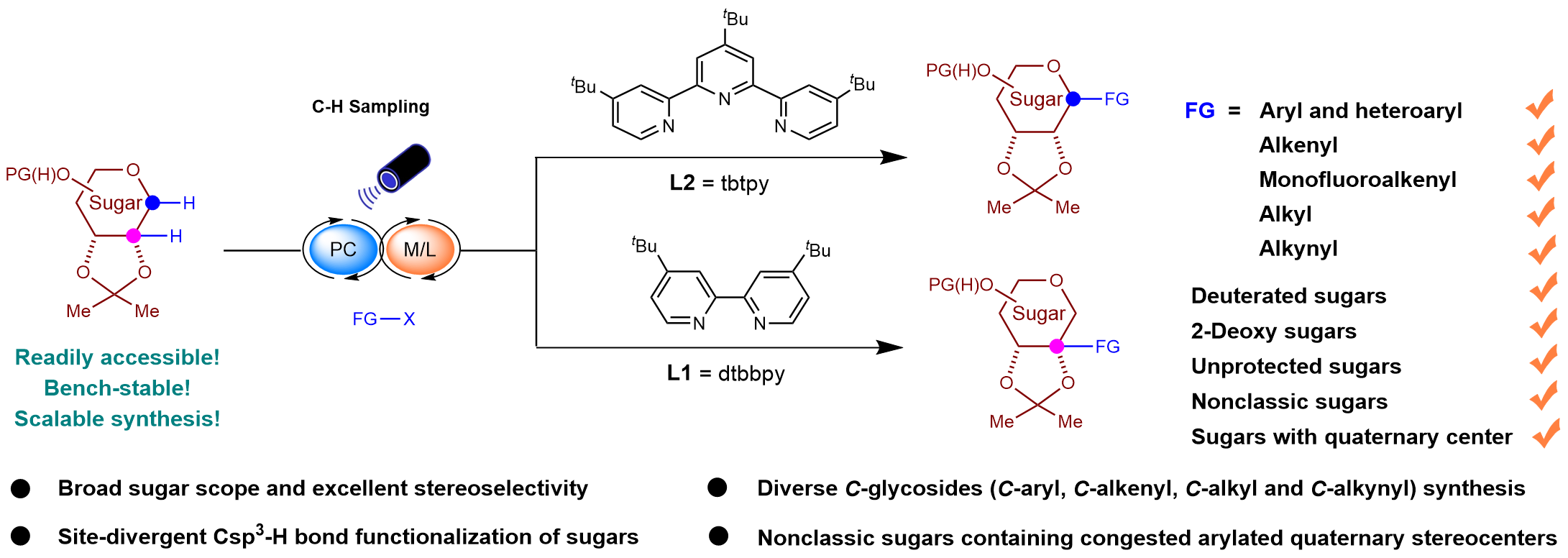
In 2023, our research team successfully achieved the enantioselective functionalization of Csp³-H bonds in oxyheterocycles using a photoinduced hydrogen atom transfer (HAT)/nickel co-catalytic strategy (J. Am. Chem. Soc. 2023, 145, 5231-5241). In this work, the Csp³-H bonds of oxyheterocycles with multiple chiral centers were subjected to stereoselective and site-selective functionalization through the manipulation of ligand configurations. Based on these findings, we hypothesized that a photo-HAT strategy could be employed to activate the C(sp3)-H bond in deoxyglycosides, followed by nickel-catalyzed modulation of site- and stereoselective glycosidic functionalization. Current glycosyl donors typically introduce reactive functional groups at position 1, thus enhancing their reactivity but also complicating their preparation. The synthesis of 1-deoxyglycosides involves the conversion of the C-O bond at position 1 into a C-H bond, a modification that significantly improves the stability of these molecules, allowing for prolonged storage under ambient conditions and enabling large-scale preparation in just two steps.
Leveraging the availability and stability of 1-deoxyglycosides as glycosyl radical precursors, we have developed a robust and versatile method for the synthesis of diverse C–R glycosides (where R represents aryl, heteroaryl, alkenyl, alkynyl, or alkyl groups) through metallaphotoredox catalysis. Furthermore, we advanced ligand-controlled, site-divergent Csp3–H bond functionalization of carbohydrates, providing an innovative approach to synthesize a variety of previously unexplored carbohydrates containing quaternary stereocenters. This reaction proceeds under mild conditions and demonstrates high stereoselectivity across a broad spectrum of glycosyl units. Moreover, the practical utility of this approach was demonstrated in the synthesis of pharmaceutically relevant molecules and complex carbohydrates.
Isotopic labeling experiments revealed that the HAT process, mediated by TBADT, is both stereospecific and reversible. Subsequent experiments investigating metal complexation showed that the Ni(0) catalyst preferentially interacts with glycosyl radicals, leading to the formation of alkyl-nickel intermediates, rather than undergoing oxidative addition to aryl bromo-substituents. The ligand effect has an important influence on the reactive site selectivity, with the higher reactivity of the tertiary carbon radical making it easier to trap and transform by Ni(0) in the presence of a bidentate nitrogen ligand. In contrast, the tridentate nitrogen ligand has a higher site resistance, which further amplifies the difference in site resistance between secondary and tertiary carbon radicals, allowing selective capture of secondary carbon radicals.
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in