Stretching muscle cells induces transcriptional and splicing transitions and changes in SR proteins
Published in Healthcare & Nursing

Key points:
- Mechanosensitive genes are part of the MAPK pathway which is activated after cell stretching
- Alternatively spliced cassette exons respond more strongly to cell stretching than other alternative splicing events
- The serine and arginine-rich proteins exhibited stronger transcriptional changes than other RNA-binding proteins
- SRSF4 phosphorylation is mechanosensitive
Introduction:
A mother and father wheel their son out of the hospital with heavy steps and despondent faces. The muscle degeneration their son is experiencing has no known cause just a known end – losing the ability to breathe because his diaphragm muscle can no longer contract. Several well-known muscle diseases have treatments that can extend a patient’s life, however, the vast number of identified muscle disorders have no known cause. For families who find out their loved one has a devastating muscle condition with no treatment options, there is no hope. Thus, it is critical to understand normal muscle development to determine what goes awry in muscle diseases.
During development, skeletal muscle matures both molecularly and mechanically. Molecularly, muscle undergoes high levels of changes in gene expression and alternative splicing programs which are necessary for proper acquisition of adult functionalities1,2. Alternative splicing is an RNA processing mechanism that increases protein diversity in Eukaryotes. Some genes that are alternatively spliced during muscle development are involved in calcium handling, biogenesis of the transverse tubules (T-tubules), and membrane trafficking2-5 Most muscle diseases are accompanied by reversions in alternative splicing programs which leads to adult muscle being poorly sustained by fetal proteins - ultimately leading to muscle atrophy and weakness.
Mechanically, skeletal muscle is an organ that must respond to force from the outside world (exercise) as well as generate force (muscle contraction). Further, muscle is sensitive to its environment – humans and mice with muscular dystrophy possess stiffer skeletal muscles which leads to muscle atrophy and weakness6. Muscle wasting and the inability to contract the muscle demonstrate how this organ is dependent on mechanical forces for proper function.
Exercise is important for improving cardiovascular health, increasing our bone density, growing our muscles, and establishing strong connections between muscles and tendons. However, in instances of muscular disease like Duchenne muscular dystrophy, amyotrophic lateral sclerosis, or myotonic dystrophy it is currently under debate if exercise can help strengthen muscles that are slowly wasting away or if it further harms the muscle at a molecular level. Thus, there is a gap of knowledge linking the mechanical and molecular components of muscle disease (Figure 1).
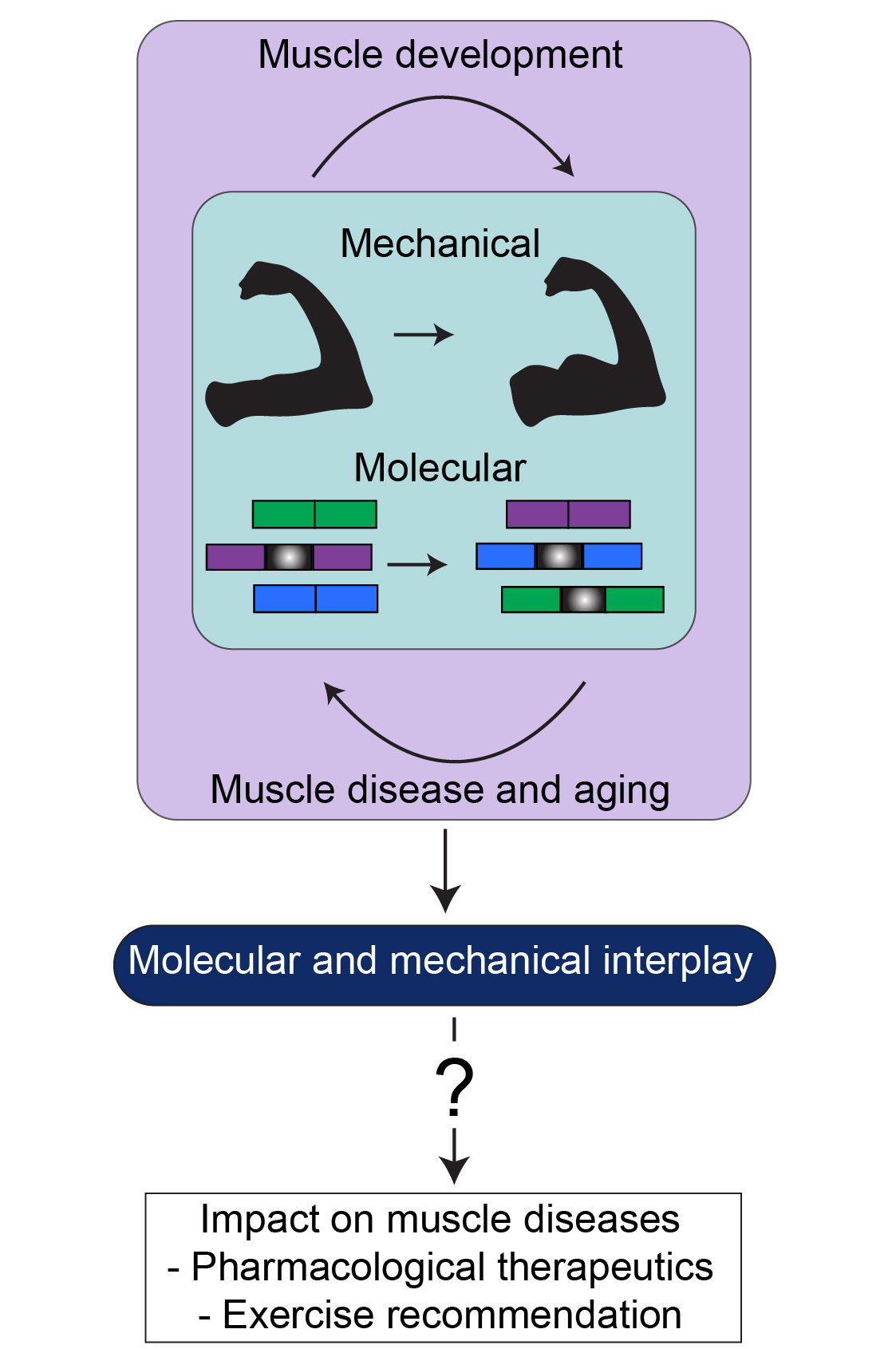
Since muscle disorders exhibit severe changes at both the molecular and mechanical level, it is critical to study them together and not independently. Further, these processes must be examined in normal skeletal muscle to accurately characterize their interplay in muscle disease. In our recently published work in Communications Biology7, we sought to unite both the molecular and mechanical elements of muscle.
Summary:
Differentiation of C2C12 mouse myoblasts into myotubes mimics the numerous splicing transitions that occur during skeletal muscle development5,8. To induce differentiation of these cells we reduced the serum concentration in the media which causes the myoblast cells to exit the cell cycle and form long, multinucleated cells which we term ‘differentiated cells.’ To mechanically stimulate cells, we utilized the Flexcell system which can stretch cells in a cyclic, equibiaxial manner which loosely mimics a hypertrophy-based exercise where many repetitions of an exercise are performed at a low weight. Using the Flexcell system, we stretched both undifferentiated myoblasts, and differentiated muscle cells for one, three, or six hours and performed deep RNA-sequencing to define the gene expression and alternative splicing networks that were responsive to mechanical forces.
After one hour of stretching the differentiated cells, some mechanosensitive genes were found to be involved in the mitogen-activated protein kinase (MAPK) pathway. The MAPK pathway was strongly activated after stretching as measured by the phosphorylation of the extracellular signal-regulated kinase (ERK). This signaling axis has been previously demonstrated to respond to mechanical forces9,10.
We also observed alternative splicing changes that were mechanosensitive. Cassette exons were more sensitive to cell stretching than other types of alternative splicing events leading us to ask which RNA-binding proteins (RBPs) may be involved in regulating mechanosensitive cassette exon splicing. The Serine-Arginine Rich (SR) family of proteins responded more strongly to stretching than other RBPs at the gene expression level. A recent paper overexpressed various SR proteins in breast acinar cells and performed RNA-sequencing to determine gene expression and alternative splicing changes11. By utilizing the data from that study, we defined a set of transcriptional and posttranscriptional targets that responded to SRSF4 overexpression as well as mechanical stretching. We next asked whether SRSF4 played a role in regulating the mechanosensitive genes and splicing events by assaying the phosphorylation of SRSF4 after mechanical stretching. SRSF4 phosphorylation decreased after one hour of cell stretching while the total SRSF4 protein levels were unchanged suggesting that stretching influences SRSF4 phosphorylation. This is the first time that an RBP has been identified as mechanosensitive in skeletal muscle cells.
The mechanosensitive genes and splicing events that overlapped with those that responded to SRSF4 overexpression were involved in the MAPK pathway. This is captivating considering how parts of this cascade have been implicated as tension sensors and we and others have found ERK phosphorylation to be mechanosensitive9,10. Interestingly, there is still no known connection between SRSF4 and ERK even though both proteins exhibit altered phosphorylation in response to cell stretching.
Future Perspective:
RBPs are implicated in almost every cellular process of skeletal muscle, however, our findings are the first to suggest that SRSF4 is involved in the response of skeletal muscle cells to mechanical forces. SRSF4 could potentially be a missing link between force transmitted to the nucleus and subsequent effects on alternative splicing. The effect of force on alternative splicing and RBPs is unknown. There is huge potential for more research in the area of the SR proteins because of the strong connection between alternative splicing and mechanotransduction in skeletal muscle. Hopefully, researching the molecular and mechanical components of skeletal muscle together will uncover mechanosensitive molecular mechanisms and could shed light on potential therapeutics to help people who suffer from muscle diseases without a known cause.
You can read the full paper here: Hinkle, E. R. et al. Stretching muscle cells induces transcriptional and splicing transitions and changes in SR proteins. Commun. Biol. 5, 987. (2022).
This work was supported by start-up funds (J.G.) and a Jefferson Pilot Award (J.G.) from The University of North Carolina at Chapel Hill, a National Institutes of Health (NIH) R01 (NIH-NIGMS R01GM130866) (J.G.), a NCTraCs Pilot Grant (550KR181805) from the National Center for Advancing Translational Sciences (NCATS) (J.G.), and a Career Development Award from the American Heart Association (19CDA34660248) (J.G.). E.R.H was supported by a NIH-NIAMS F31 predoctoral fellowship (F31AR077381) and a NIH-NIGMS training award (5T32 GM007092). We gratefully acknowledge the technical support from the High Throughput Sequencing Facility at The University of North Carolina at Chapel Hill, which is supported by the University Cancer Research Fund, Comprehensive Cancer Center Core Support grant (P30-CA016086), and UNC Center for Mental Health and Susceptibility grant (P30-ES010126). M.C. was supported by a NIH-NIAMS F31 predoctoral fellowship (1F31HL145983-01). J.M.T. was supported by a NIH-NHLBI R01 (1R01HL142879). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any other funding source. We thank the Bioinformatics Core at The University of North Carolina at Chapel Hill for their continued help during this project and their jUNCtion program for analyzing data. We acknowledge the support of the Genetics and Molecular Biology Curriculum (GMB) at The University of North Carolina at Chapel Hill. We especially thank Dr. Keith Burridge for numerous valuable discussions regarding this project.
References:
- Merkin, J., Russell, C., Chen, P. & Burge, C. B. Evolutionary dynamics of gene and isoform regulation in mammalian tissues. Science. 1593–1599 (2012).
- Brinegar, A. E. et al. Extensive alternative splicing transitions during postnatal skeletal muscle development are required for Ca 2+ handling. Elife 6, e27192 (2017).
- Giudice, J., Loehr, J. A., Rodney, G. G. & Cooper, T. A. Alternative splicing of four trafficking genes regulates myofiber structure and skeletal muscle physiology. Cell Rep. 17, 1923–1933 (2016).
- Fugier, C. et al. Misregulated alternative splicing of BIN1 is associated with T tubule alterations and muscle weakness in myotonic dystrophy. Nat. Med. 17, 720–725 (2011).
- Hinkle, E. R. et al. Alternative splicing regulation of membrane trafficking genes during myogenesis. RNA 28, 523–540 (2022).
- Engler, A. J. et al. Myotubes differentiate optimally on substrates with tissue-like stiffness. J. Cell Biol. 166, 877–887 (2004).
- Hinkle, E. R. et al. Stretching muscle cells induces transcriptional and splicing transitions and changes in SR proteins. Commun. Biol. 5, 987. (2022).
- Smith, J. A. et al. FXR1 splicing is important for muscle development and biomolecular condensates in muscle cells. JCB 219, e201911129 (2020).
- Martineau, L. C. & Gardiner, P. F. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J. Appl. Physiol. 91, 693–702 (2001).
- Wretman, C. et al. Effects of concentric and eccentric contractions on phosphorylation of MAPKerk1/2 and MAPKp38 in isolated rat skeletal muscle. J. Physiol. 535, 155–164 (2001).
- Park, S. H. et al. Differential functions of splicing factors in mammary transformation and breast cancer metastasis. Cell Rep. 29, 2672-2688.e7 (2019).
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in