Synthesis of a glycan hairpin
Published in Chemistry

The intricate folding of biopolymers plays a crucial role in enabling their diverse and sophisticated functionalities. Taking cues from nature and guided by the principle “form follows function”, scientists have successfully engineered peptide and nucleic acid sequences that can adopt specific three-dimensional shapes to carry out targeted functions. However, the exploration of autonomously folding synthetic glycans, with their complex structures and lack of design principles, has remained largely uncharted territory.
The project started back in 2019 when we decided to take on the challenge of folding a glycan. We realized that polysaccharides adopt fairly often helical conformations in aqueous solution but no report existed of polysaccharide β-sheets. In proteins, β-sheets are essential secondary structural elements and key drivers of the formation of fibrous protein assemblies such as amyloids. Peptide hairpins represent the simplest unit of extended β-sheets. Hairpin structural elements have attracted a broad interest as self-assembling nanomaterials and as scaffolds for asymmetric catalysis. We asked ourselves if we could reproduce a hairpin structural element using a carbohydrate backbone. How would such a structure appear, and what remarkable characteristics could it exhibit? The first step was creating one from scratch.
We quickly realized that designing such a folded structure wasn’t an easy task. We needed to identify oligosaccharide sequences to compose two linear strands and a connecting turn unit.
First, for the stacking strands of the parallel glycan hairpin structure, we found inspiration in the remarkable properties of cellulose, a polysaccharide renowned for its structural strength and abundance in nature.1 Composed of a repeating sequence of β-1,4-D-glucose units, cellulose exhibits a rigid rod conformation in solution and tends to interact with other cellulose chains through numerous hydrogen bonds and hydrophobic interactions. We had learned this through previous projects in which we realized that even short oligomers behave much like longer cellulose chains.2,3
The second step was identifying a sequence that could adopt a turn unit conformation to hold the two cellulose strands and position them in a parallel arrangement. Identifying the right turn unit was the most challenging part of the design and it took us some time to explore different sequences. To understand which could be the right sequences, we used Molecular Dynamics (MD) simulations and simulated a wide range of structures that we first sketched on paper. Ultimately, we stumbled upon an interesting trisaccharide motif, the Lewis X trisaccharide. This motif is present in a wide range of mammalian glycans and consists of an N-acetyl D-glucosamine core to which a β-1,4-D-galactose and a α-1,3-L-fucose branch out.4 This special sequence folds into a stable turn-like conformation in solution stabilized by hydrophobic interactions and by a non-conventional hydrogen bond between the two branches.5 Two simple modifications to this natural motif were necessary to transform it into an oligosaccharide turn unit. D-Galactose was replaced by D-glucose and L-fucose by L-rhamnose. This modified version possessed two hydroxyl groups ideally oriented in space for attaching the two cellulose strands. Combining the newly designed turn unit with the cellulose strands could, in our hypothesis, generate a glycan hairpin.
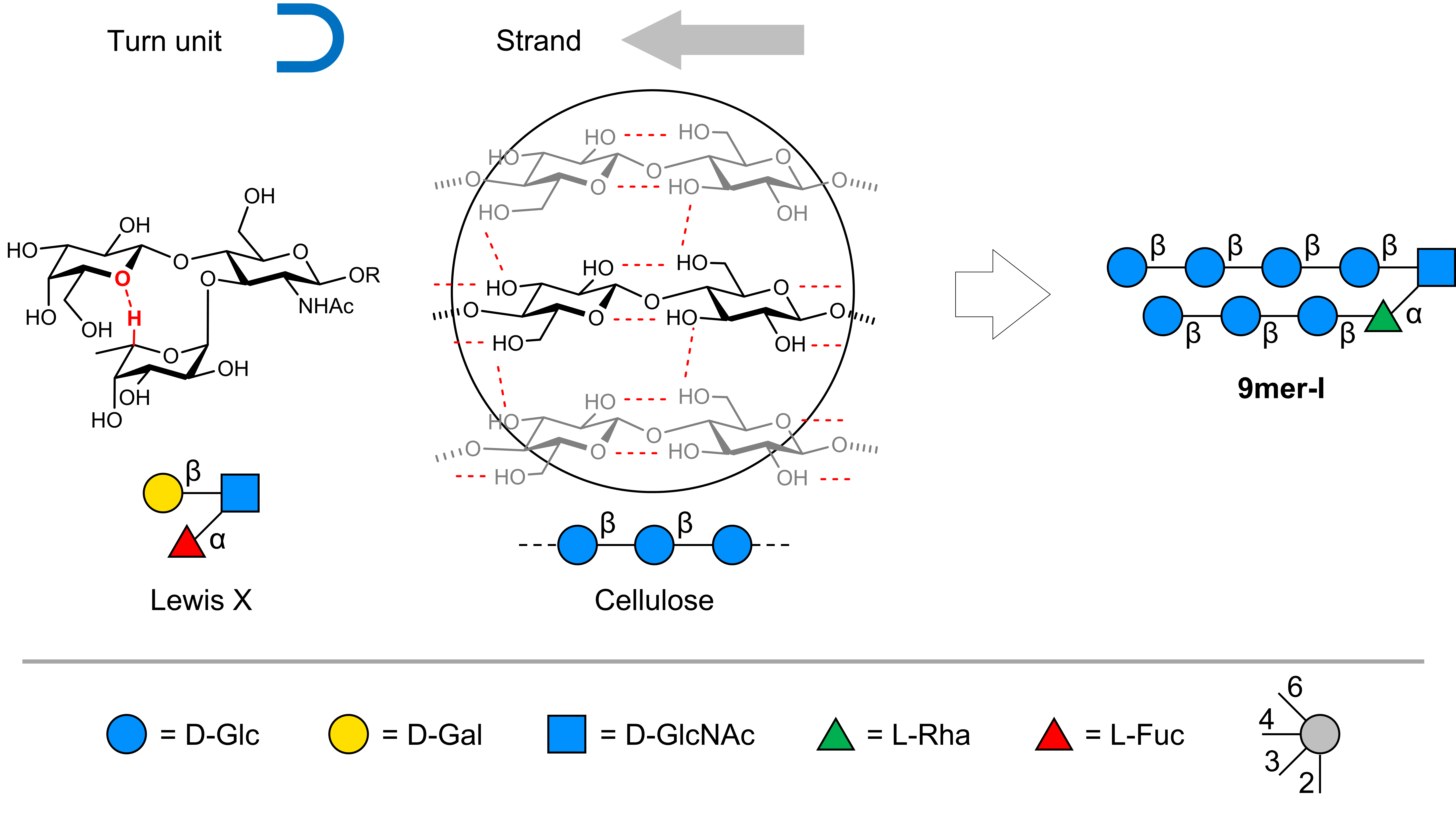
In order to validate our hypotheses, we had to synthesize the glycan hairpin. To construct the target molecules, we used Automated Glycan Assembly (AGA), an automated solid-supported synthetic method to construct glycans starting from protected monosaccharide building blocks. With some synthetic optimization, this technology allowed for the rapid and precise construction of several analogs including the designed turn unit and the 9mer glycan hairpin.
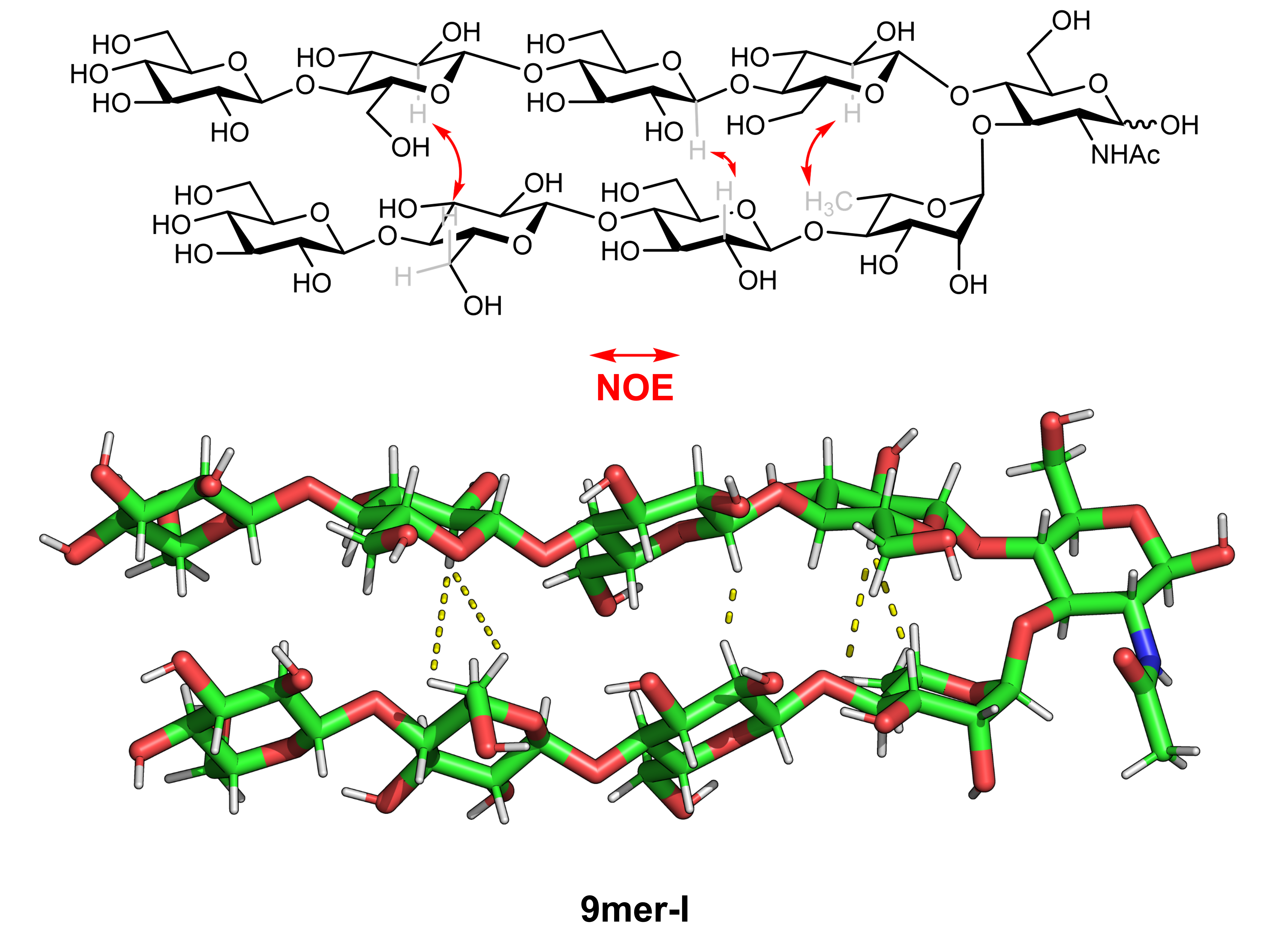
To reveal the conformation that these compounds adopt in solution, we employed nuclear magnetic resonance (NMR) based methods. NMR enables the study of the shape and dynamic behavior of glycans in solution but comes with its own complexities. Analyzing glycans using NMR can be challenging due to the presence of numerous similar protons in the molecule, resulting in overlapping peaks that introduce ambiguity. As we moved from simple trisaccharides to nonasaccharides the NMR analysis became increasingly more complex. To our rescue came Dr. Ana Poveda and Prof. Jesús Jiménez-Barbero applying 2D Nuclear Overhauser Effect Spectroscopy (NOESY) and Diffusion Ordered Spectroscopy (DOSY) for structural analysis. Combining these powerful structural analysis techniques with synthetic 13C-labelled analogs was the key to extract the information that we wanted. By mapping all the NOEs, we confirmed the 3-D model calculated from MD simulations suggesting the glycan adopts a conformation in which the two strands are in close spatial proximity.
Our work illustrates that glycan sequences capable of adopting specific secondary structure motifs can be designed, expanding the range of foldamer scaffolds. With a vast pool of monosaccharides available, there are likely numerous structural motifs beyond the commonly known ones that remain to be discovered. Controlling the conformation of glycans holds potential for creating materials with predictable functions and properties, with applications in catalysis and nanotechnology and the recent improvements in glycan synthesis offer opportunities to explore these exciting avenues.6
References
- Jarvis, M. Cellulose Stacks Up. Nature 2003, 426 (6967), 611–612.
- Yu, Y.; Tyrikos-Ergas, T.; Zhu, Y.; Fittolani, G.; Bordoni, V.; Singhal, A.; Fair, R. J.; Grafmüller, A.; Seeberger, P. H.; Delbianco, M. Systematic Hydrogen-Bond Manipulations To Establish Polysaccharide Structure-Property Correlations. Angew. Chemie Int. Ed. 2019, 58 (37), 13127–13132.
- Fittolani, G.; Vargová, D.; Seeberger, P. H.; Ogawa, Y.; Delbianco, M. Bottom-Up Approach to Understand Chirality Transfer across Scales in Cellulose Assemblies. J. Am. Chem. Soc. 2022, 144 (27), 12469–12475.
- Aeschbacher, T.; Zierke, M.; Smieško, M.; Collot, M.; Mallet, J.; Ernst, B.; Allain, F. H. ‐T.; Schubert, M. A Secondary Structural Element in a Wide Range of Fucosylated Glycoepitopes. Chem. - A Eur. J. 2017, 23 (48), 11598–11610.
- Zierke, M.; Smieško, M.; Rabbani, S.; Aeschbacher, T.; Cutting, B.; Allain, F. H.-T.; Schubert, M.; Ernst, B. Stabilization of Branched Oligosaccharides: Lewis x Benefits from a Nonconventional C–H···O Hydrogen Bond. J. Am. Chem. Soc. 2013, 135 (36), 13464–13472.
- Wu, Y.; Qiu, Y.; Feng, Y.; Stoddart, J. F. Automating Glycan Assembly in Solution. ACS Cent. Sci. 2022, 8 (10), 1369–1372.
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in