Taming Titania: Facile synthesis of mesoporous TiO2 architectures with tunable configurations and nanometer precision
Published in Materials and Civil Engineering
Corresponding authors: Kun Lan & Dongyuan Zhao
Corresponding institutions: Inner Mongolia University & Fudan University, China
For decades, mesoporous TiO2 has been a workhorse material in science and technology, prized for its use in everything from solar cells and sensors to catalysts. Our team, like many others, is fascinated by mesoporous this material to give it an ordered mesoscopic structure. Such a design dramatically increases surface area, enhancing its performance by improving mass diffusion and making reaction sites more accessible.
However, working with titanium dioxide at the nanoscale presents a classic challenge. The chemical precursors used to build TiO2 structures are incredibly reactive. Trying to assemble them into delicate, ordered architectures is often too fast and chaotic, making precise control over the final shape, pore size, and crystal structure extremely difficult. To unlock the full potential of mesoporous TiO2, we knew we needed to find a way to tame this unruly assembly process.
Our breakthrough, which is the focus of our new Nature Protocols paper, came from shifting our perspective. Instead of trying to control the entire chaotic process at once, we developed a stepwise self-assembly strategy. The key was to first prepare stable, uniform "building blocks" in the form of a "micelle hydrogel". We use a common polymer, F127, to form spherical micelles that gently encapsulate the titanium precursors in a carefully controlled acidic solution, thus avoiding the usual flash hydrolysis. This process yields a hydrogel full of stable, dissociated F127/TiO2 composite micelles, each about 6-12 nm in diameter, which serve as the perfect building blocks for constructing a diverse range of nanostructures in a second, highly controlled step.
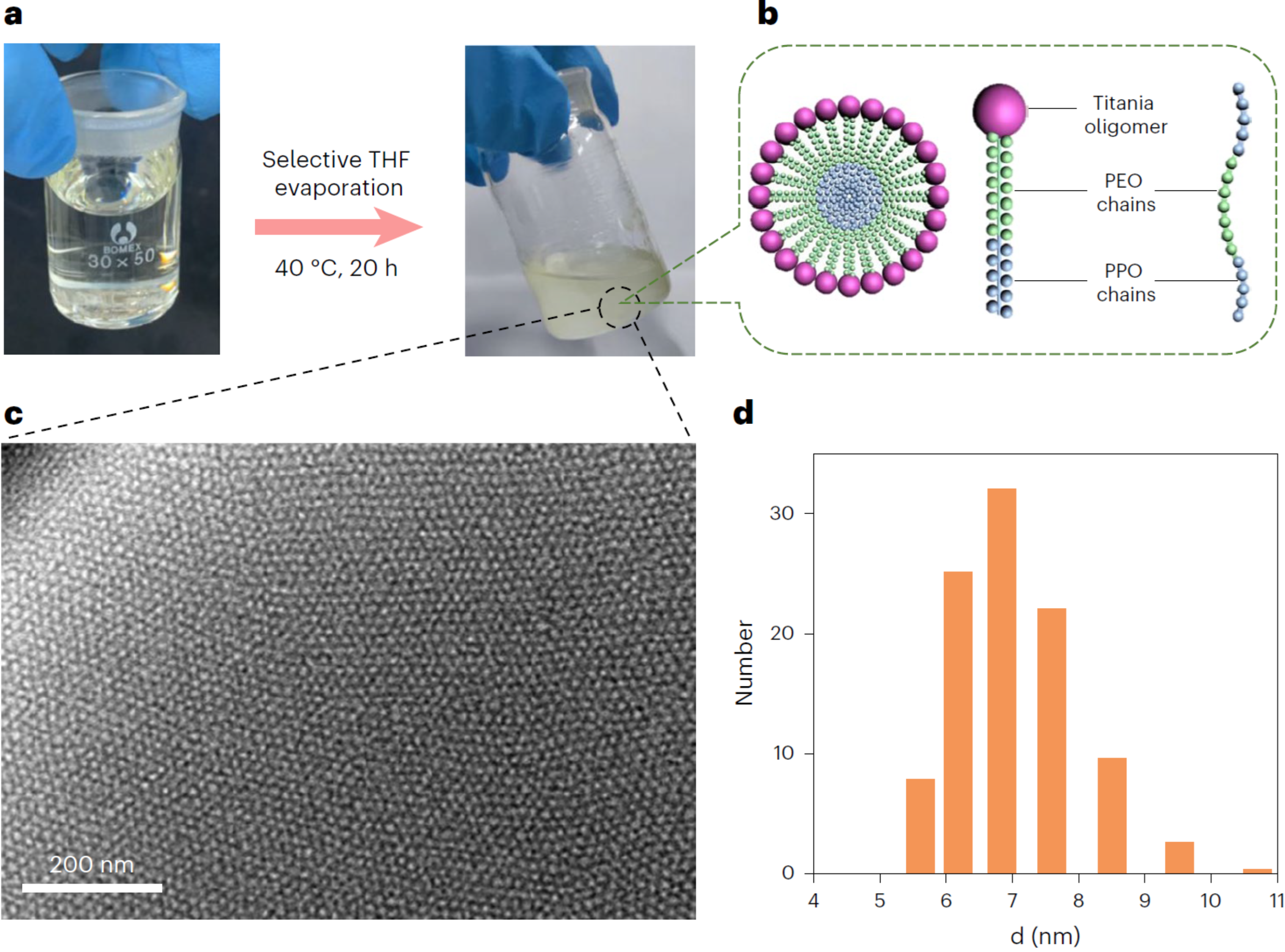
Fig. 1 Preparation and characterization of the primitive F127/TiO2 composite micelle hydrogel.
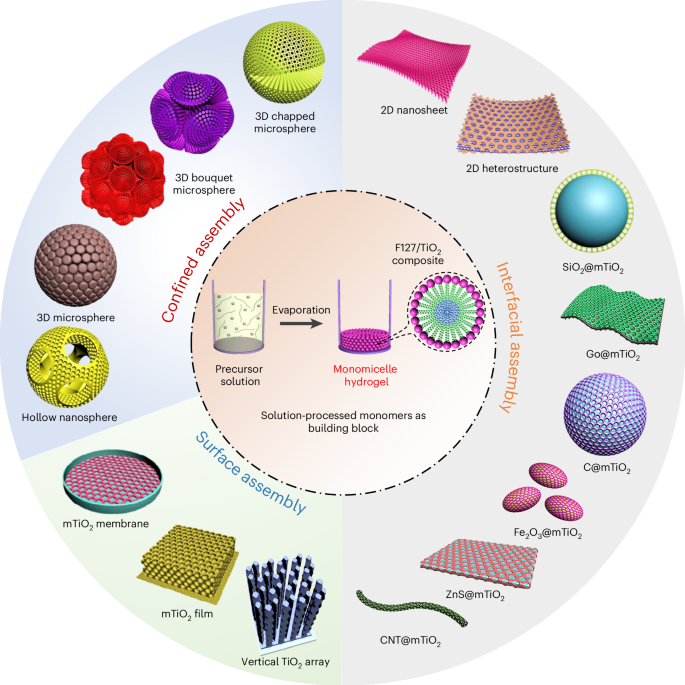
The true power of this method is its versatility. Once we have the hydrogel, we can guide the assembly of these building blocks into a stunning variety of architectures simply by changing the external conditions.
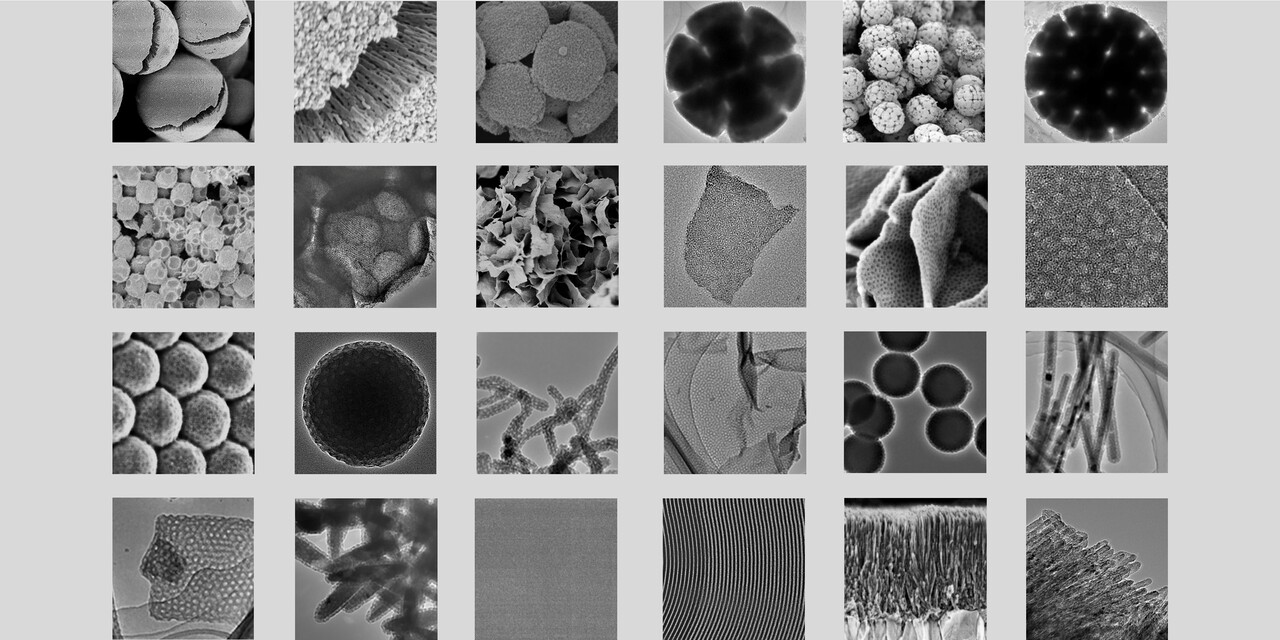
- By assembling them in a confined emulsion, we can create intricate 3D architectures like radially-oriented microspheres or even complex, beautiful "bouquet-posy-like" superstructures.
- By guiding the assembly at a liquid-liquid interface, we can produce ultrathin, 2D monolayered nanosheets only a few nanometers thick.
- By applying the micelle dispersion to a solid substrate, we can grow highly ordered membranes with tunable thickness and crystal structures.
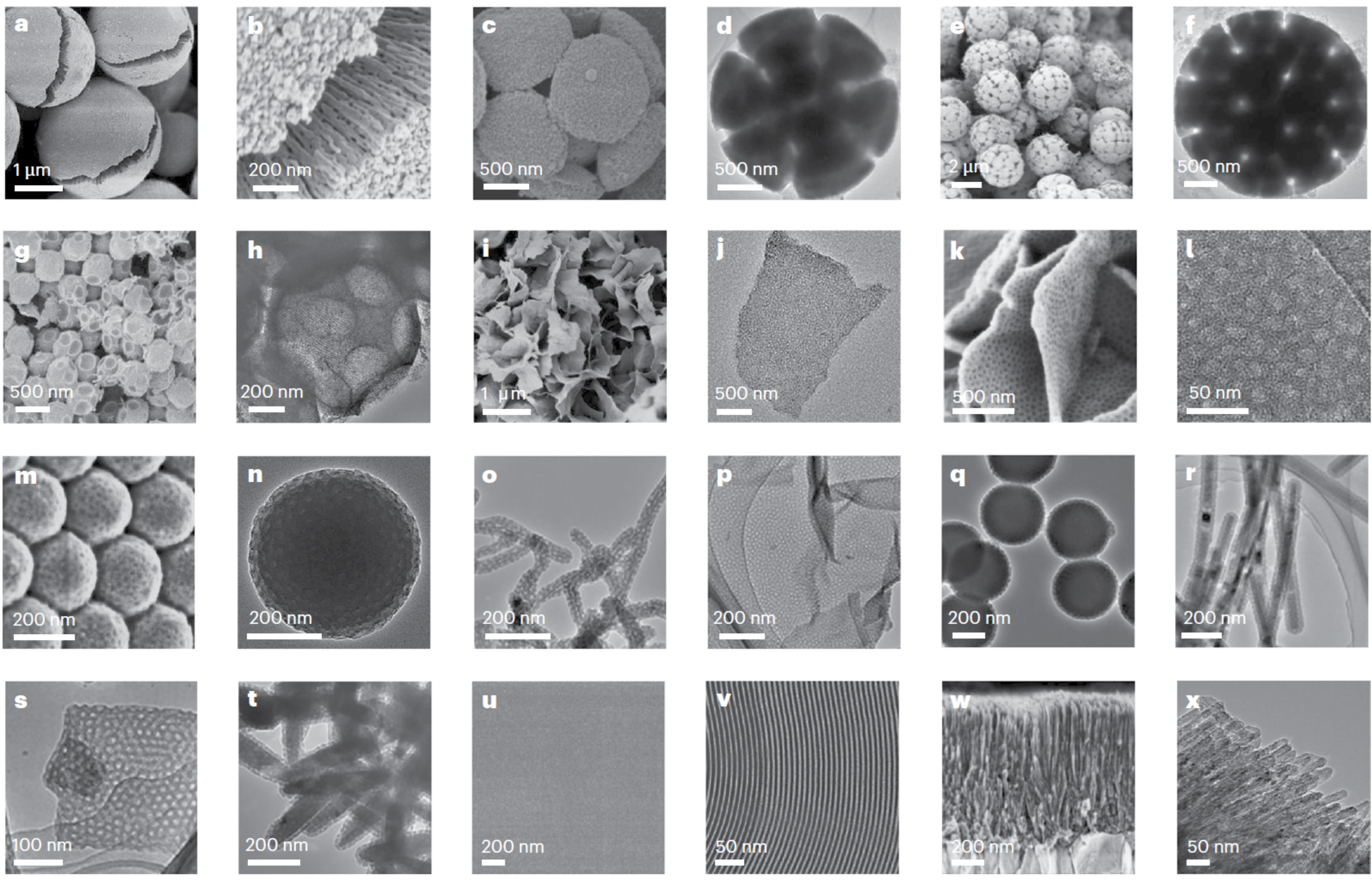
Fig. 2 Summarized electron microscopy (EM) images of ordered mesoporous TiO2 materials in versatile architectures.
This protocol gives researchers some unprecedented methods of control, allowing for the precise tuning of not just the overall shape, but also pore size, grain size, and even the crystalline phase (anatase or rutile) of the final TiO2 materials. The applications are immediate and exciting. We've already demonstrated their potential in creating high-performance anodes for sodium-ion batteries and highly efficient photocatalysts for hydrogen generation.
For more details, please see our full paper, "Facile synthesis of mesoporous TiO2 architectures with tunable configurations and nanometer precision" in Nature Protocols.
(https://doi.org/10.1038/s41596-025-01175-3)
Follow the Topic
-
Nature Protocols

This journal publishes secondary research articles and covers new techniques and technologies, as well as established methods, used in all fields of the biological, chemical and clinical sciences.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in