Targeting the Cell Cycle’s Achilles Heel: WEE1 Inhibition Offers New Hope for Hard-to-Treat Gynecological Cancers Driven by Cyclin E1 Expression
Published in Cancer and General & Internal Medicine

Gynecological cancers, including ovarian and uterine cancers, have a significant impact on women's health. While treatments like chemotherapy can initially be effective, many patients experience recurrence, and outcomes haven't improved significantly in decades. This is especially true for aggressive forms of these cancers, like high-grade serous ovarian carcinoma (HGSOC) and uterine serous carcinoma (USC), when they are resistant to traditional chemotherapies. But what if there was a way to target a specific weakness in these cancer cells, making them more vulnerable to treatment? This is exactly what we have been exploring.
The Cell Cycle and Cyclin E1
To understand this new approach, it’s important to know a bit about the cell cycle. Cells go through a tightly regulated cycle of growth, and key proteins, including cyclins and cyclin-dependent kinases (CDKs), act as the "gears and levers" moving cells through each phase of the cycle (G1, S, G2 and M). One of these key players is Cyclin E1, which teams up with CDK2 to accelerate the cell from the G1 phase into the S phase where DNA replication occurs (Figure 1A).
In many cancers, particularly gynecological cancers, Cyclin E1 is overproduced, leading to an overactive Cyclin E1/CDK2 complex. This speeds up the cell cycle and can cause problems because cells do not have sufficient time to repair damaged DNA generated from a regular replication cycle or exogenous stimuli. As a result, these cancer cells become highly dependent on the G2/M checkpoint to manage and enable repair of DNA damage, creating a potential vulnerability to drugs targeting this checkpoint.
WEE1: The Cell Cycle's Brake
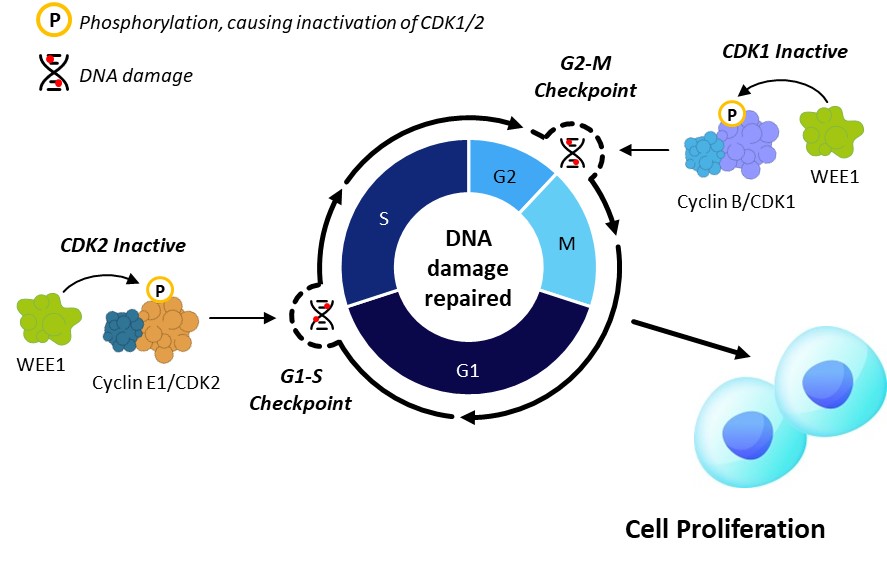
Now, imagine that the cell cycle has brakes and accelerators. WEE1 kinase works as the brake in the cell cycle while Cyclin E1 acts as the accelerator as described above. WEE1 arrests the cell cycle by inhibiting both CDK1 and CDK2 and is involved in regulating several stages of cell cycle, especially the checkpoints at G1/S and G2/M boundary (Figure 1A). This allows cells time to repair DNA damage before proceeding with cell division and is particularly important in cells with high levels of baseline replication stress and DNA damage, such as those with high level of Cyclin E1 protein. Blocking WEE1 will slam the brakes and force these cells to enter mitosis without appropriately repairing damaged DNA, ultimately leading to cell death (Figure 1B).
Figure 1. Azenosertib Mechanism of Action – Inhibitor of WEE1, a Master Cell Cycle Regulator
A. Normal Cell Cycle Regulation
B. WEE1 Inhibition by Azenosertib in Cyclin E1-Positive Cancer Cells
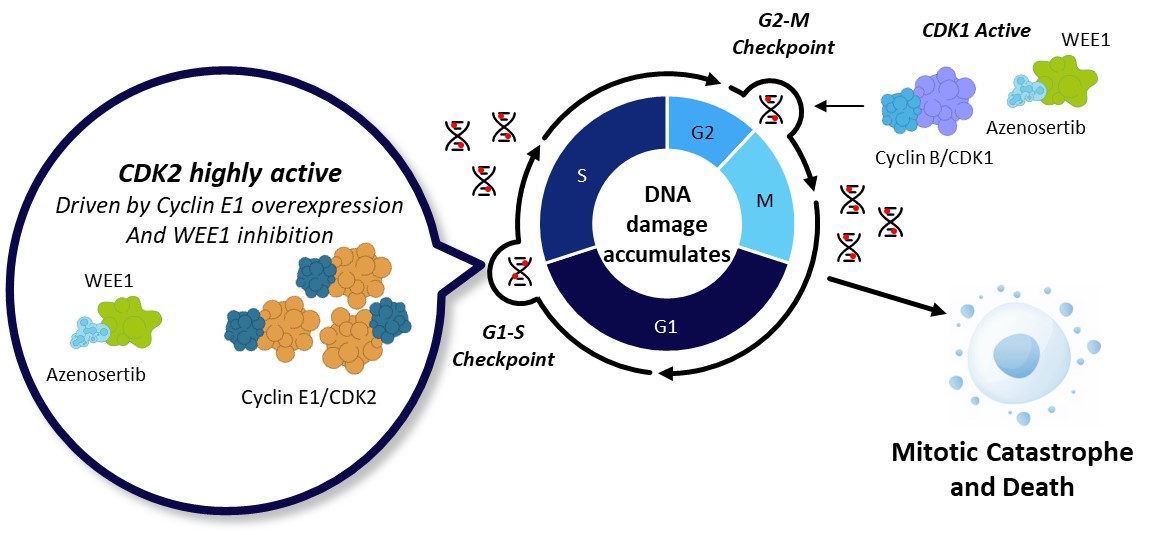
Figure 1. A. In normal cells, WEE1 kinase acts as a brake to halt the cell cycle. Following DNA damage, WEE1 phosphorylates and inactivates Cyclin/CDK complexes at both G1-S and G2-M checkpoints to halt the cell cycle and allow for repair. Upon DNA repair, cells progress through the cell cycle and proliferate. B. Cyclin E1 overexpression (with or without CCNE1 gene amplification) increases CDK2 activity and accelerates G1/S transition, resulting in increased replication stress and DNA damage which relies on G2/M checkpoint for DNA repair. WEE1 inhibition by azenosertib further activates CDK2 and CDK1, bypassing both G1-S and G2-M checkpoints, leaving insufficient time for the cells to repair DNA damage. Consequently, cells undergo mitotic catastrophe and cell death.
The Key Idea: Targeting Cyclin E1-positive Cancers with WEE1 Inhibitor Azenosertib
Based on the above knowledge, we hypothesized that cancer cells with high levels of Cyclin E1 (Cyclin E1-positive), which already have increased replication stress, would be particularly sensitive to WEE1 inhibition. Using a novel selective WEE1 inhibitor, azenosertib, we tested this idea in cell lines as well as mouse models harboring tumors derived from patients or human cancer cell lines.
What we found is that high Cyclin E1 levels (Cyclin E1 positivity) were associated with enhanced sensitivity to azenosertib. Ovarian cancer cell lines with high levels of Cyclin E1 showed greater sensitivity to azenosertib than those with low levels. When we artificially increased the amount of Cyclin E1 protein in cancer cells, their sensitivity to azenosertib also increased. As we hypothesized, azenosertib exacerbated the already existing replication stress and DNA damage in Cyclin E1-positive cells beyond their repair capacity, and induced cell death. These observations were well translated into animal models where azenosertib demonstrated greater anti-tumor activity against Cyclin E1-positive tumors derived from USC patients or ovarian cancer cell lines.
Early Clinical Evidence: A Promising Start
Excitingly, we also observed early clinical evidence from an ongoing clinical trial that patients with Cyclin E1-positive cancers responded well to azenosertib treatment. Among three highlighted partial responders with Cyclin E1-positive tumors, two were confirmed to have CCNE1 gene amplification (the gene encoding Cyclin E1 protein), while one did not. This suggests that elevated Cyclin E1 protein level − regardless of the CCNE1 amplification status − may serve as a predictive marker for response to azenosertib.
Azenosertib and Chemotherapy: A Powerful Combination
Combination therapy, which combines two or more therapeutic agents, is widely used to treat cancers to enhance therapeutic benefits and potentially overcome or reduce drug resistance. Chemotherapy is the most common treatment for gynecological cancers, exerting its effect by inducing DNA damage, disrupting DNA replication, or impairing microtubule dynamics essential for cell division. This provides a strong rationale for combining these agents with azenosertib. Indeed, we observed azenosetib worked synergistically with three common chemotherapy drugs that represent different chemotherapy classes: gemcitabine (antimetabolite), carboplatin (platinum), and paclitaxel (taxane). This combination strategy broadens the potential patient population and offers a powerful approach to overcoming resistance.
Why is This Important and Future Directions:
High levels of Cyclin E1 protein in cancer cells are often linked to poor outcomes and resistance to treatment. Our study, however, identifies this as a specific weakness for WEE1 targeting and showcases the promising anti-tumor effect of the selective WEE1 inhibitor azenosertib in both preclinical and clinical settings. By targeting WEE1 in Cyclin E1-positive cancers, azenosertib represents a tailored therapeutic option for these hard-to-treat tumors. It also suggests a way to potentially overcome chemotherapy resistance, which is a major challenge in cancer treatment.
The application of azenosertib in Cyclin E1-positive gynecological cancer patients is being further investigated. Ongoing research aims to further explore its potential, including its combinations with other therapies, to improve clinical outcomes for patients with gynecological cancers and beyond.
Please visit Zentalis Pharmaceuticals at https://www.zentalis.com/ for the latest clinical updates on azenosertib in Cyclin E1-driven cancers
Follow the Topic
-
npj Precision Oncology

An international, peer-reviewed journal committed to publishing cutting-edge scientific research in all aspects of precision oncology from basic science to translational applications to clinical medicine.
Related Collections
With Collections, you can get published faster and increase your visibility.
Tumor-type-agnostic biomarkers and treatments in oncology
Publishing Model: Open Access
Deadline: Mar 05, 2026
Emerging adjuvant and neo-adjuvant treatment approaches in solid tumors
Publishing Model: Open Access
Deadline: Mar 30, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in