The bare retinal chromophore from visual photoreceptors shows an ultrafast photoisomerization dynamics
Published in Chemistry

The interaction of molecules with light is central to vital activity of living organisms. Photosynthesis, vision in vertebrates, solar energy harvesting and conversion are all triggered by absorption of a photon. These processes are remarkably efficient, and many scientific efforts are being made to elucidate the role played by the protein environment in their primary events, which occur on a timescale down to sub-picoseconds. Light-absorbing molecules, the so-called chromophores, lie at the heart of the photoactive proteins. Vision is an important example, where a single chromophore, 11-cis retinal in the protonated Schiff-base form, is responsible for absorption of light in visual Opsin proteins.
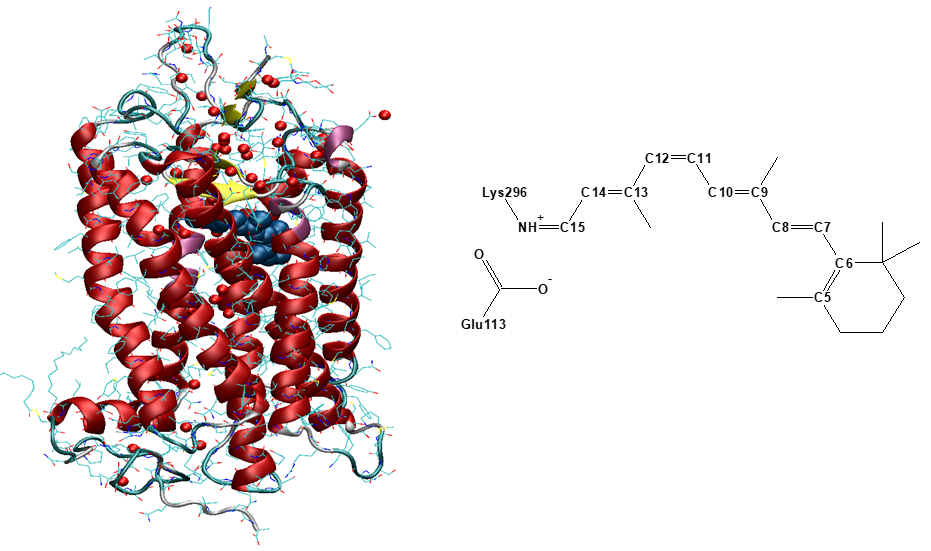
The primary step in vision, the 11-cis to all-trans photoisomerization inside the protein, is one of the fastest processes in nature, where nuclear rearrangements along the reaction pathway occur within hundreds of femtoseconds. On the other hand, the reaction is about ten times slower in solutions. A fundamental question is: do proteins speed up the reaction, and if so how? – or does the protein scaffold simply protect the chromophore and let it respond to what may be considered its "natural" ultrafast timescale?
This question has remained unanswered since George Wald was awarded the Nobel Prize in 1967 for his pioneering work on color vision. It is normally assumed that the role of the protein is to speed up the reaction. Here, we have tested this hypothesis directly and performed the first time-resolved pump-probe experiments on the protonated Schiff-base retinal in vacuo. From the experimental side, the problem arises when dealing with positively charged chromophores, since a proper molecular response should be found that is sensitive to a probe pulse. Molecular anions have been considered for years in other laboratories, where detection of photoelectrons is readily available. Reports of dynamics in cations are, on the other hand, rare.
We have developed a new 2D time-resolved method to address both excited-state lifetimes and ground-state recovery of molecular anions as well as cations. This work is built upon our previous research (Phys. Rev. Lett., 2016, 117, 243004, J. Am. Chem. Soc., 2017, 139 (25), pp 8766–8771), which combines the SAPHIRA ion storage ring with a femtosecond laser system (Aarhus University). Identification of the absorption of two consecutive (pump and probe) photons is provided by the time, at which the molecular action (dissociation) eventually takes place when molecules return to the ground state; hence time in two dimensions – the statistical fragmentation time and the pump-probe delay.
By combining measurements with high-level ab initio calculations carried out using high performance computing resources at Lomonosov Moscow State University, we are now able to provide a real time reference for photoisomerization. As we demonstrate here, the intrinsic photoisomerization may indeed be very fast – hundreds of femtoseconds for the 11-cis isomer, and slow – several picoseconds for the all-trans isomer. This is traced to different energy barriers in the excited state of these isomers. Importantly, we show that the ultrafast photoresponse of visual photoreceptors is directly related to the intrinsic properties of the retinal chromophore, thus shedding new light on the role of the protein environment and improving our knowledge about the functioning of retinal-containing proteins.
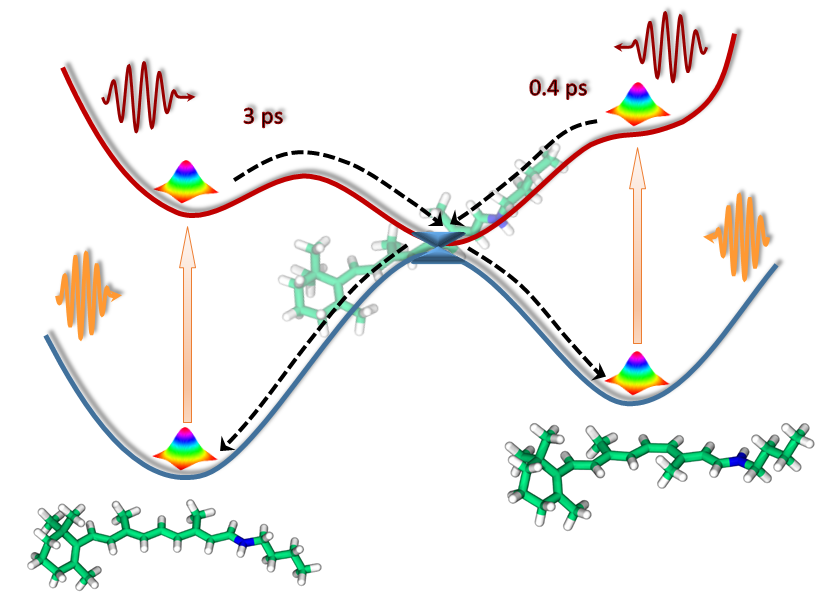
We believe that knowledge at the local molecular level is imperative, like the significance of knowing the molecular structure of DNA for genetics. This may not be enough to fully account for the functioning of entire proteins; however, our results certainly open up a new physical dimension to protein research through establishing an essential reference for dissecting the complexity of biosystems and providing a link between the photophysics and photochemistry of photoactive proteins and their chromophores.
Read more details in our publication:
H.V. Kiefer, E. Gruber, J. Langeland, P.A. Kusochek, A.V. Bochenkova, L.H. Andersen
Intrinsic photoisomerization dynamics of protonated Schiff-base retinal
Nature Communications 10, article number 1210 (2019); DOI:10.1038/s41467-019-09225-7
The text is written by Anastasia V. Bochenkova and Lars H. Andersen
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in