The mechanics of crocodile head scale development
Published in Ecology & Evolution, Physics, and Cell & Molecular Biology


- A juvenile (2 years old) Nile crocodile.
-
Credits: M. C. Milinkovitch & A. Debry
© michel.Milinkovitch@unige.ch — University of Geneva, Switzerland
From the tough scales of reptiles to the soft fur of mammals, vertebrates possess a remarkable variety of skin features that enable them to thrive in diverse environments. These features, known as skin appendages, serve multiple purposes, such as protecting the body from environmental challenges, regulating temperature, and providing camouflage. To perform their functions effectively, these skin features must be arranged in specific patterns. For example, birds have feathers arranged in neat rows to optimize flight (1), while snakes exhibit scales in a nearly perfect hexagonal pattern, aligned with their muscles and ribs (2), which facilitates their extraordinary ability to move across various substrates.
It is well-established that the spatial patterning of skin appendage primordia — called placodes (3, 4) — is self-organized through interactions between activatory and inhibitory morphogens, forming a so-called Turing reaction-diffusion system (5-7). In other words, the resulting self-organised “polka-dot” arrangement of gene expression provides a template for where skin features such as feathers, scales or hairs will grow (1, 3, 4, 8).
However, crocodiles are a spectacular exception to this paradigm. Unlike the scales of other reptiles and birds, and unlike their own body scales, the irregularly shaped head scales of crocodiles are not individual developmental units patterned by reaction-diffusion among gene products. Instead, these scales seem to form through a mechanical process (9). Yet, the exact nature of this process has remained elusive for more than a decade. In our new study published this week in Nature (10), we finally uncover the answer to this mystery. Using advanced techniques such as in-ovo drug treatment of crocodile embryos, cutting-edge 3D microscopy, and state-of-the-art computer simulations, we reveal that these head scales are patterned through a purely mechanical self-organising compressive-folding process. Let me take you through the long journey behind this discovery.
It all began about 15 years ago. While sampling blood from a Nile crocodile for a comparative genomic study, I was struck by the pattern of irregular scales on the face and jaws of that magnificent animal. First, the size and shape of these scales are highly variable across the head. The positions and sizes of these scales also differ from one individual to another, and even between the left and right sides of the same individual. Second, many edges are incomplete (i.e., unconnected), as if some scales never got fully delimited. I decided to quantitatively characterise this pattern. To do so, I searched for a device capable of scanning multiple crocodiles and reconstructing the three-dimensional geometry and colour texture of their heads. I could not find any off-the-shelf equipment that offered sufficient resolution. Evidently, such equipment did not exist on the market. Thus, with three PhD students (Antonio Martins, Michel Bessant and Liana Manukyan), we developed R2OBBIE-3D, our in-house fast and high-resolution system for reconstructing surface geometry and colour texture (11). R2OBBIE-3D combines an industrial robotic arm, a high-resolution digital color camera, an basket of high-intensity LEDs and state-of-the-art 3D-reconstruction approaches, allowing us to accurately reconstruct (with a resolution of about 50 microns) colored 3D model of nearly any object. You can see R2OBBIE-3D at work in this video.
In 2013, we published an article (9) in which we demonstrated that crocodile head scales form through the emergence, propagation and interconnection of skin grooves without the involvement of genes expressed in feather, scale or hair placodes. Hence, crocodile head scales are not developmental units. Instead, they are polygonal domains of highly-keratinised skin whose size and shape depend on how these grooves interconnect. The final pattern — with incomplete edges and a substantial number of 90° edge junctions — is reminiscent of material cracking in a tensile stress field. One speculative explanation for this superficial similarity was that skin growth could be coupled to mechanical tension produced by the rapid growth of the crocodile embryonic jaw skeleton: local folds, nucleated by tension-driven growth, would cause tensile stress to redistribute and accumulate at their tips, leading to their propagation in a cracking-like manner.
A couple of years later, upon gaining access to a few additional crocodile embryos, we identified that cell proliferation was not substantially increased at fold tips during head scale patterning. Thus, one alternative possibility was that the skin actually folds in a compressive stress field because it grows faster than the underlying skull to which it is firmly connected.
Notably, tissue buckling from ‘constrained growth’ is thought to shape other biological forms, including the intricate folds of the human brain (12), the villi (finger-like projections) of the human and chicken gut (13), and the small polygons on the glabrous skin of the rhinarium (or naked nose) of dogs, cows and ferrets (14). Interestingly, we have recently shown that, for this latter example, the polygonal form of the pattern is imposed by the underlying polygonal pattern of blood vessels that act as ‘mechanical positional information’ (14).
Given the central difficulties of obtaining and experimenting with crocodile embryos, it took us years to make substantial progress on the crocodile project. The year 2020 was a turning point, as we gained the opportunity to access some additional Nile crocodile eggs. I then assigned to Gabriel Santos-Durán, a bright post-doctoral fellow in my laboratory, the very tricky tasks of (a) setting up a technique for intravenous injection in crocodile embryos, and (b) identifying a drug that would affect skin growth during head-scale development. The latter experiment would identify which of the tension-driven or the compression-driven hypothesis could be safely rejected. For example, accelerating skin growth would reduce versus increase folding under the first versus the second hypothesis, respectively. Not only did Gabriel successfully set up the injection protocol, but he also identified EGF (the epidermal growth factor protein) as a relatively non-toxic drug that produced embryos with increased folding of their head skin, thereby invalidating the hypothesis of tension-driven patterning and supporting the compression-driven alternative process.
I then took the risk in 2022 to import a large number of Nile crocodile eggs from a South-African breeding farm. While we were bracing for the possibility of a disastrous reception (a massive omelet), the eggs safely arrived in my laboratory in Geneva. That’s when Rory Cooper, a fantastic post-doctoral fellow in my lab, entered the scene. Rory fine-tuned the injection protocol (performing three in ovo EGF injections at three-day intervals at the onset of skin folding), identified a clear dose effect of EGF in increasing skin growth and keratinisation, and showed that the treatment was very reproducible. Remarkably, as we increased the dose of EGF, we observed the presence of an extensive labyrinthine pattern of convoluted folds across the jaws of the embryo, instead of the normal polygonal scales. When we performed two more injections to maintain exacerbated epidermal growth until hatching, the newborn crocodile exhibited a strongly convoluted pattern on its head. Clearly, these results demonstrated that crocodile head scales develop through compressive folding and that EGF treatment exacerbates this folding.

- An EGF-treated Nile crocodile hatchling.
Credits: Rory Cooper & M. C. Milinkovitch
© michel.Milinkovitch@unige.ch — University of Geneva, Switzerland.
Next, Rory scanned crocodile embryos with a cutting-edge light sheet microscope that we acquired for 3D imaging of very large samples. These data allowed Ebrahim Jahanbakhsh, a brilliant computer engineer in my lab, to build a mathematical growth model incorporating the key elements that we suggest determine the mechanical patterning of crocodile head scales. These elements include the geometry of the skin (such as the spatial variation of epidermal and dermal thicknesses) and underlying bone, as well as the relative growth of the two skin layers. Importantly, the model developed by Ebrahim also allows for introducing anisotropic material such as collagen fibres. Collagen is a key determinant of tissue-specific material properties, but it is notoriously difficult to image in 3D. Fortunately, with a talented PhD student (Grigorii Timin) in my lab, we recently solved this issue by developing a simple and robust method of whole-mount collagen staining with the ‘Fast Green’ dye that provides unmatched visualization of collagen 3D network architecture (15).
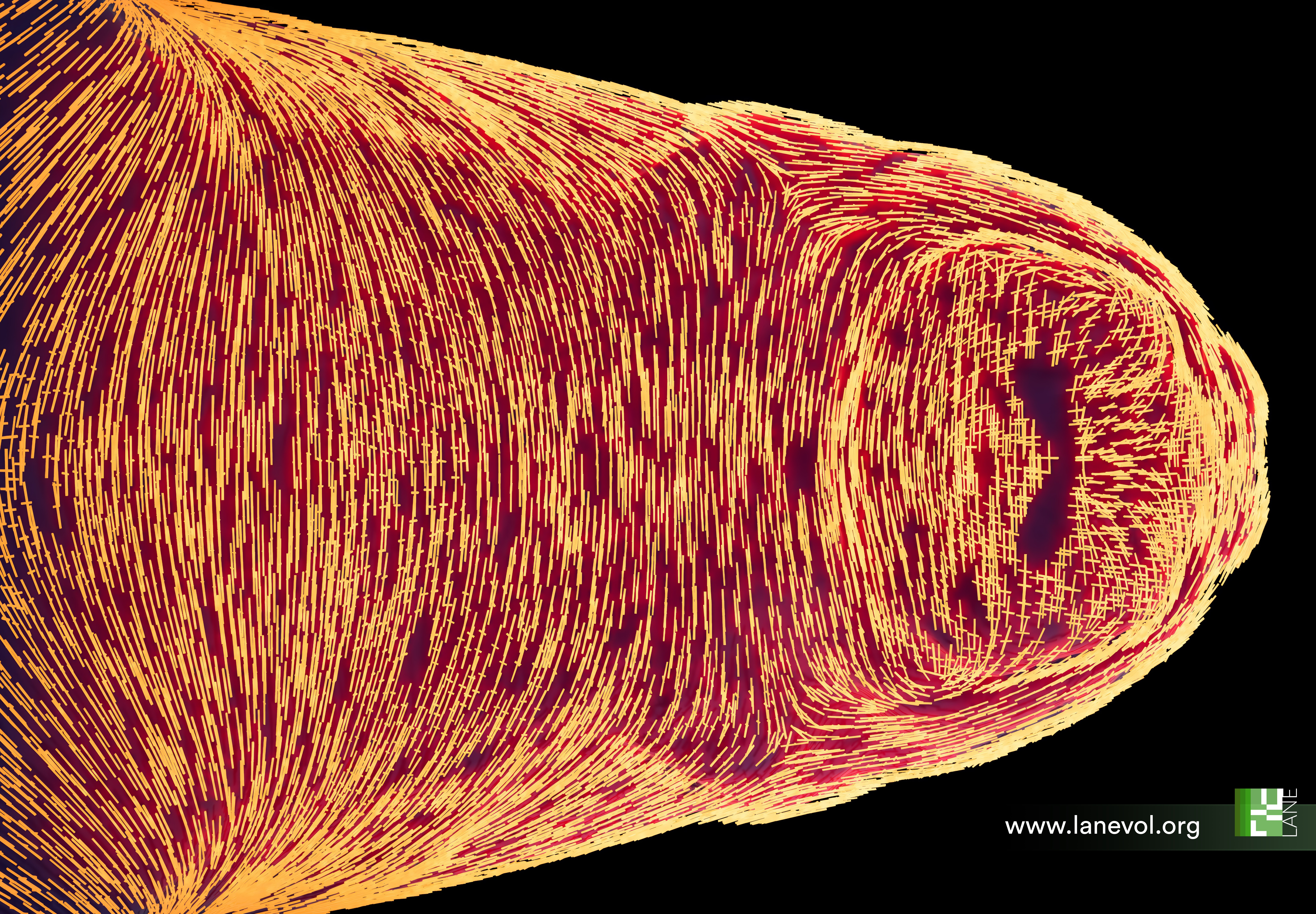
- Spatial variation of collagen fibre orientations identified by 3D fast Fourier transform of light-sheet microscopy images.
Credits: E. Jahanbakhsh & M. C. Milinkovitch
© michel.Milinkovitch@unige.ch — University of Geneva, Switzerland.
Remarkably, our computer growth simulations accurately reproduced the natural head scale arrangement of Nile crocodiles, which includes elongated units on top of the jaws and smaller polygons on the sides. In addition, increasing both skin growth and stiffness in our simulations replicated the labyrinthine (‘brainy’) folding pattern seen in our EGF-treated embryos. These results show that the polygonal arrangement of crocodile head scales can indeed emerge from a purely mechanical system of constrained skin growth, i.e., in the complete absence of self-organising gene interactions.
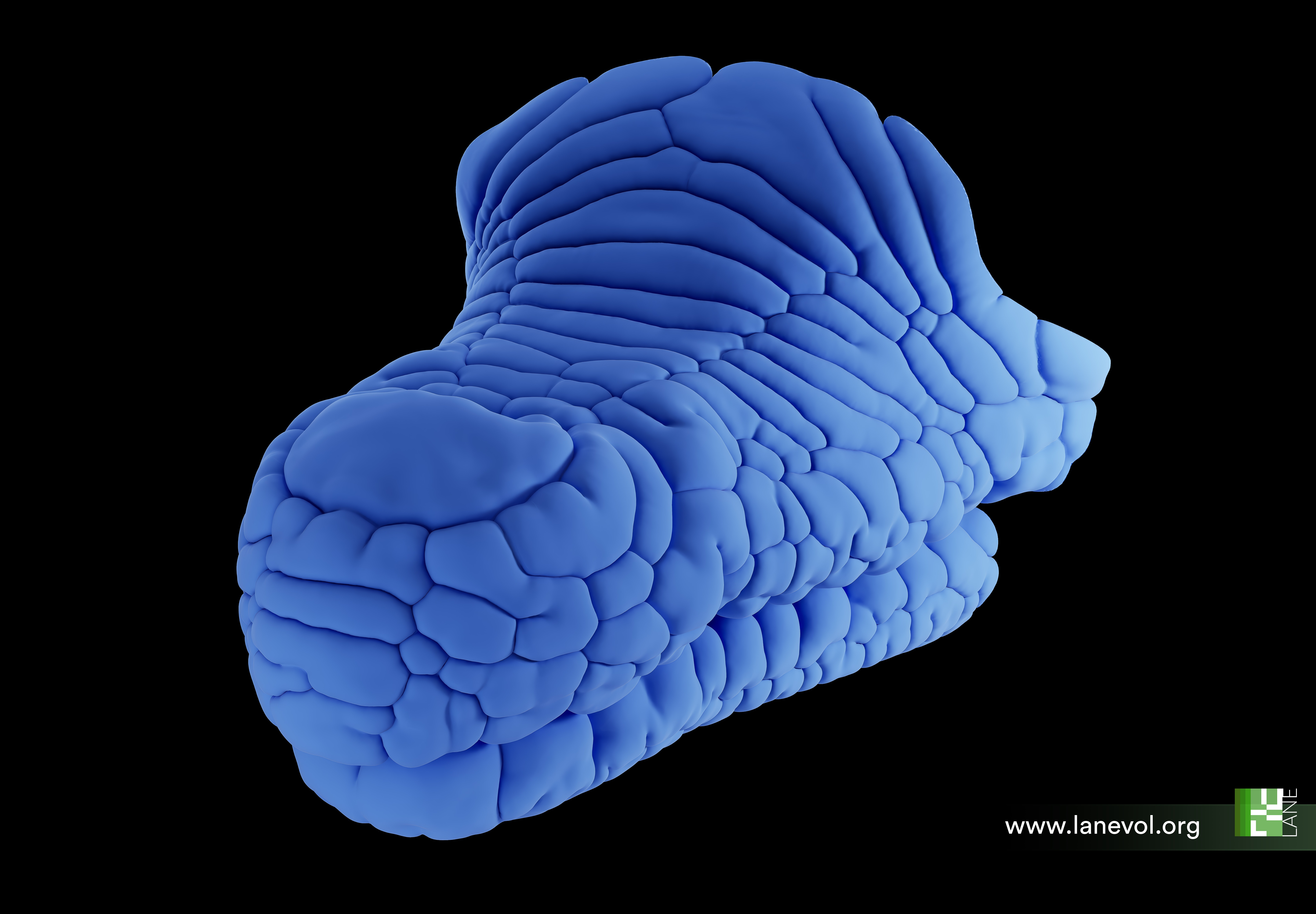
- Our mechanical growth simulation model recapitulates normal crocodile head scale patterning.
Credits: E. Jahanbakhsh & M. C. Milinkovitch
© michel.Milinkovitch@unige.ch — University of Geneva, Switzerland.
But our study does not stop there. While understanding the purely mechanical development of the head scale pattern in Nile crocodiles is exciting, deciphering how such a process can evolve — and therefore explain the variety of patterns observed in different crocodilian species — is even more intriguing. As the convoluted labyrinthine pattern of folds (generated by discrete injections of large doses of EGF) is very dissimilar to the natural polygonal pattern of Nile crocodiles, I wondered what would happen if, instead of injecting three large doses of EGF at three-day intervals at the onset of skin folding, we continuously injected low doses of EGF until the embryo hatched at approximately day 90. This would experimentally simulate a slightly increased growth of the epidermis over the whole period of head scale development. Would the hatchling exhibit a labyrinthine pattern of folds?
As the current techniques at our disposal do not allow us to perform such an experiment, I asked Rory to treat some eggs as usual but, instead of collecting the embryos, to let the crocodiles hatch four weeks after the EGF treatment had finished. Most remarkably, these newly hatched crocodiles no longer displayed a fully labyrinthine pattern, as it had partially relaxed into a pattern of smaller polygons resembling that of another crocodilian species — the spectacled caiman. This result is particularly important because it demonstrates that promoting skin growth and differentiation during embryonic development can alter the hatchling head scale pattern from that of a Nile crocodile to that of another species. Hence, I hypothesized that variations in embryonic growth and material properties of skin layers might provide a simple evolutionary mechanism explaining the diversity of head-scale patterns among crocodilian species.
To investigate this conjecture, we resorted to Ebrahim’s computer model. First, we recapitulated the results obtained with hatched EGF-treated crocodiles: exacerbating epidermal growth in our simulations generated a labyrinthine pattern, but switching back to normal skin growth caused the convoluted pattern to relax into a pattern of smaller-than-normal polygons resembling those of a caiman. Second, we produced a so-called ‘theoretical morphospace’ of skin folding patterns by smoothly varying the relative epidermal versus dermal growth rates and elastic moduli. This morphospace included the whole diversity of observed crocodilian head scale patterns, including those of Nile crocodiles, marsh crocodiles, spectacled caimans and American alligators. Therefore, shifts in multiple molecular components of an intricate Turing reaction-diffusion system (7) are not required to explain the evolution of head scale patterns in crocodilians. Instead, slight variations in skin growth and/or stiffness can clearly explain these macroevolutionary changes. In this case, mechanics makes evolution much simpler !
Check out our (hopefully appealing) 3D imaging data and numerical simulations in the following video summarising our results:
- English version: https://www.youtube.com/watch?v=vuvUrAJTg2U
- A shorter English version: https://youtu.be/FD2pqEDLw4s?si=fUGFrjt7q2ZP9DYr
- A French version: https://www.youtube.com/watch?v=7eSPgCm_60I
- Versions in other languages (Spanish, German, Japanese and Chinese) will be made available in January 2025.
References
1. W. K. W. Ho et al., Feather arrays are patterned by interacting signalling and cell density waves. PLoS biology 17, e3000132 (2019).
2. A. C. Tzika, A. Ullate-Agote, S. Zakany, M. Kummrow, M. C. Milinkovitch, Somitic positional information guides self-organized patterning of snake scales. Sci Adv 9, eadf8834 (2023).
3. N. Di-Poï, M. C. Milinkovitch, The anatomical placode in reptile scale morphogenesis indicates shared ancestry among skin appendages in amniotes. Science Advances 2, e1600708 (2016).
4. R. L. Cooper et al., An ancient Turing-like patterning mechanism regulates skin denticle development in sharks. Science Advances 4, eaau5484 (2018).
5. A. M. Turing, The Chemical Basis of Morphogenesis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 237, 37-72 (1952).
6. S. Kondo, T. Miura, Reaction-Diffusion Model as a Framework of Understanding Biological Pattern Formation. Science (New York, N.Y.) 329, 1616-1620 (2010).
7. M. C. Milinkovitch, E. Jahanbakhsh, S. Zakany, The Unreasonable Effectiveness of Reaction Diffusion in Vertebrate Skin Color Patterning. Annu Rev Cell Dev Biol 39, 145-174 (2023).
8. S. Sick, S. Reinker, J. Timmer, T. Schlake, WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 314, 1447-1450 (2006).
9. M. C. Milinkovitch et al., Crocodile Head Scales Are Not Developmental Units But Emerge from Physical Cracking. Science 339, 78-81 (2013).
10. G. N. Santos-Durán, R. L. Cooper, E. Jahanbakhsh, G. M. Timin, Michel. C., Self-organised Patterning of Crocodile Head Scales by Compressive Folding. Nature, 11 December 2024.
11. A. F. Martins, M. Bessant, L. Manukyan, M. C. Milinkovitch, R(2)OBBIE-3D, a Fast Robotic High-Resolution System for Quantitative Phenotyping of Surface Geometry and Colour-Texture. PLoS One 10, e0126740 (2015).
12. T. Tallinen et al., On the growth and form of cortical convolutions. Nature Physics 12, 588-593 (2016).
13. A. E. Shyer et al., Villification: How the Gut Gets Its Villi. Science 342, 212-218 (2013).
14. P. Dagenais et al., Mechanical positional information guides the self-organized development of a polygonal network of creases in the skin of mammalian noses. Curr Biol, (2024).
15. G. Timin, M. C. Milinkovitch, High-resolution confocal and light-sheet imaging of collagen 3D network architecture in very large samples. iScience 26, 106452 (2023).
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
"Wow, this is super interesting! It's crazy how crocodile head scales develop through a natural, self-organizing process using compressive folding. The way mechanical forces and skin growth work together to create such unique patterns across different crocodile species really shows how evolution mixes biology and physics in the coolest ways!"