The memory of salt: searching for ancient microbial life biosignatures on Earth and Mars
Published in Chemistry, Earth & Environment, and Microbiology
Ancient Salts and the Search for Life: Cracking Open the Crystal Time Capsules
Imagine a grain of salt. Common, translucent, easily overlooked. Now imagine that inside this grain lies a droplet of water (fluid inclusion) older than humanity itself, perhaps even the remnants of an ancient microbial life. This isn’t science fiction. It’s one of the most intriguing real-life puzzles in extremophile microbiology and astrobiology.
On Earth, salt flats and evaporite formations, such as halite, represent not only the memory of ancient seas, but also the habitat of salt-loving microorganisms called halophiles. Trapped within microscopic fluid inclusions, halophiles, and the biomolecules they leave behind may act as biosignature of ancient life. Microorganisms have been recovered from halite fluid inclusions dating back hundreds of millions of years, raising controversies regarding the actual age of the microorganisms found. On Mars, satellites and rovers have revealed vast mineral deposits that hint at long-lost hypersaline lakes. If life ever emerged on the red planet, these salts might be where it left its last trace.
In short, salt is more than just seasoning. Crystals may be archives of molecular evidence, potentially preserving biosignatures from worlds that may no longer be habitable. But if these archives are to be properly read, the scientific community must overcome the gaps in analytical compatibility, chemical understanding, and interpretative frameworks. In particular, deepening our grasp of how different salt compositions in both terrestrial and Martian context, and (in the case of Mars) salt interaction with solar UV radiation influence biosignature preservation.
But here is the catch: unlocking these salty secrets is anything but simple.
Beyond the Vault: Salt’s Impact on Biosignatures
Salt crystals and their fluid inclusion are more than passive containers. Their chemical composition and physical properties actively shape the fate of any entrapped biological material. Whether in their liquid form as brines or as solid crystals after evaporation, salt systems actively influence molecular stability, the rates of degradation, and chemical reactivity.
But despite their central importance, salts remain understudied, leaving many questions unanswered:
Can biomolecules really be preserved for millions of years inside halite? How does salt chemistry and solar radiation interact to preserve, or destroy, cell fragments? And critically: how can we tell an ancient biological signal from a false positive?
And why are the unanswered? Because salt is a nightmare in the lab.
A Sticky Situation for Science
Analysing biomolecules in brines can be compared to navigating a biochemical minefield, where each ionic interaction can obscure, destabilize, or mimic the very signatures we seek to detect. Brines interfere with almost every tool in the biochemical toolbox. They clog up mass spectrometers, scramble chromatographic separations, and disrupt fluorescent assays. Simple steps like extraction, separation, or concentration often require extensive optimization to prevent loss or degradation of the very molecules under investigation. So, in short, working with brines is not for the faint-hearted.
A salty tango: a tale of salt and radiation
In our experimental study investigating the impact of Early Earth and Mars-analogue brines on Halobacterium salinarum cell envelope extracts, this knowledge gap was directly addressed. Two main effects were examined: the chaotropicity of the brines and, relative to Martian-type brines, their UV-induced photochemical reactivity.
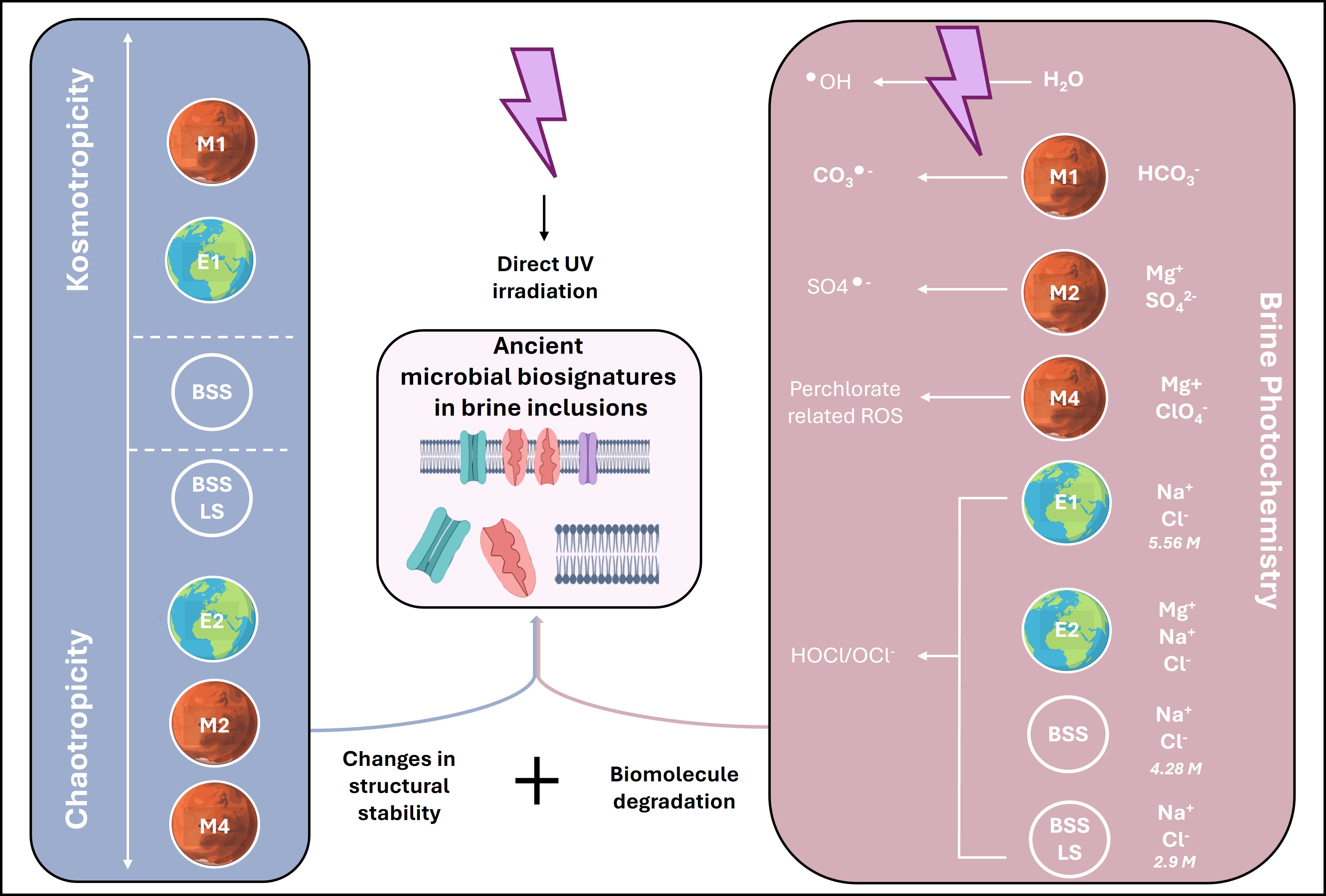
Salt Chemistry as a Double-Edged Sword
Salts themselves exert powerful, and sometimes unpredictable, effects on biosignature stability. Ionic composition is everything. Some salts, such as sodium chloride (NaCl), can be relatively benign. Others, like magnesium perchlorate, are aggressive oxidants capable of rapidly degrading proteins and lipids.
Brine chemistry influences biomolecular stability through chaotropic and kosmotropic effects, terms that describe whether a solute promotes or disrupts macromolecular order. Chaotropic ions (like Mg²⁺, ClO₄⁻) destabilize protein folding, leading to denaturation, where kosmotropic ions (like SO₄²⁻, Na⁺) do the opposite, enhancing structural integrity. But in mixed brine solutions, especially those simulating Mars’ complex geochemistry, these effects are not simply additive or linear. One brine may preserve membrane proteins, another may degrade them, even if both appear similar on paper. Moreover, preservation does not depend only on ion type—but on concentration, pH, viscosity, water activity, and temperature.
This diversity of brine behaviour makes it difficult to generalize. A fluid inclusion from early Mars may have exceptional biosignature preservation, or none at all, depending on localized mineralogy and environmental history. Each salt deposit must therefore be evaluated individually, with tailored experimental simulations that recreate both chemical and radiative stresses.
Photochemistry: The Unseen Destroyer
Another major player in the salt-biosignature interplay is photochemistry. On Earth, the ozone layer protects surface environments from high-energy UV radiation. On Mars, no such shield exists. Evaporates on the Martian surface are bombarded by UV-B and UV-C, which trigger the formation of reactive oxygen species (ROS) and radicals. These products such as hydroxyl radicals, superoxides, hypochlorous acid, are potent agents of organic degradation.
However, the extent of this degradation is strongly brine dependent. In laboratory simulations, some salt solutions produce abundant ROS under UV exposure but cause surprisingly low molecular damage, possibly due to secondary protective effects. Others, with modest ROS levels, induce substantial degradation, perhaps because of higher accessibility of biomolecules to attack. The interaction between brine photochemistry and molecular structure is complex, nonlinear, and brine specific.
The implication is profound: the success or failure of biosignature detection in a given sample may hinge not just on whether life was present, but on the obscure photochemical dynamics of the brine that once surrounded it.
Moving Forward: Building the Experimental Framework
Our study, which evaluated how brine chaotropicity and UV photochemistry impact the preservation of cell envelope proteins as biosignatures, addressed a critical knowledge gap. As methods are being made compatible to hypersaline solutions, an experimental framework predicting which environments are most likely to preserve molecular biosignature of past microbial life, need to be created. To achieve this, a wider diversity of salt compositions, longer preservation timescales, and a broader range of organisms, particularly those adapted to the specific salt composition expected on Mars, must be incorporated.
Moreover, future Mars missions should integrate these insights into site selection and sampling strategy. Not all salts are equal, and not all biosignatures will be preserved in the same way. The history of the brine, the identity of the microorganism, and the mode of preservation all matter. With limited return sample capacity, prioritizing salt deposits with favourable preservation conditions would maximize the chance of detecting ancient Martian life, if it ever existed.
So, to conclude, keep in mind, just as we look deep into the Universe to glimpse the distant past, peering into salt crystals may open a door to ancient worlds, preserving traces of life that time alone could not erase.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in