The PICCOS trial of experimental spray chemotherapy
Published in Cancer, Biomedical Research, and General & Internal Medicine
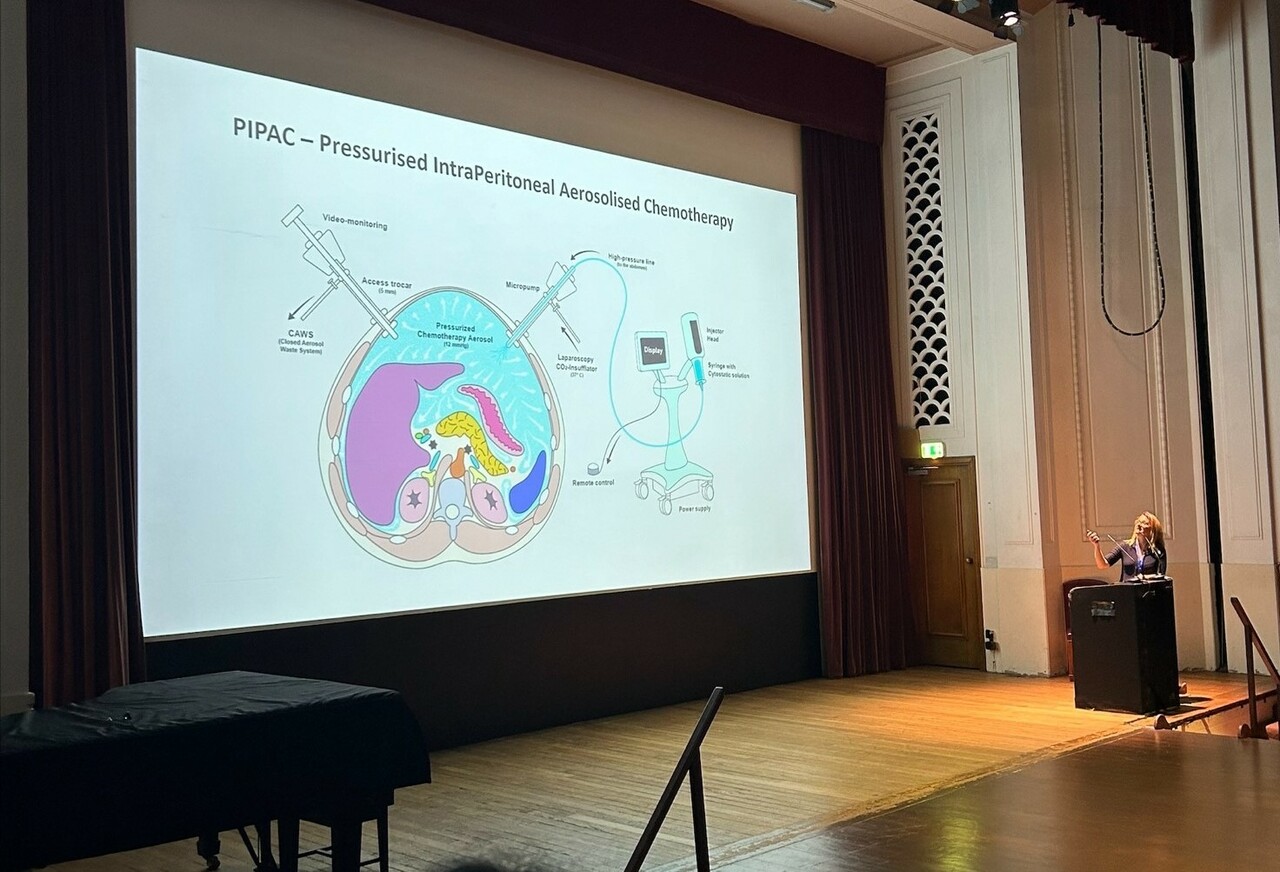
Background
Peritoneal metastases are secondary tumours that can develop around the abdomen. They cause unpleasant symptoms with relatively rapid patient decline due to the new tumour growth. This clinical trial aims to address the lack of treatment options for people with peritoneal metastases as they are difficult to treat with conventional anti-cancer drugs. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a new method of delivering anti-cancer drugs as an aerosol to the peritoneal cavity during keyhole surgery. It’s been shown to deliver higher doses of chemotherapy drugs directly to the tumour sites in comparison to conventional therapy given via a drip (intravenously) with fewer side effects demonstrated due to less chemotherapy circulating in the bloodstream. 
The PIPAC in the management of cancer of the colon, ovary and stomach (PICCOS for short) trial aims to develop high-level evidence for the efficacy of this drug delivery method to these patient groups. This will determine whether it improves survival and quality of life (QoL) in patients with peritoneal metastases. It is the first randomised controlled trial (RCT) in the UK to assess the effects of this treatment and the impact on QoL for the patients. Based on guidance from the National Institute for Health and Care Excellence (NICE), it can only be offered to patients in the UK within this research clinical trial setting. Since these tumours are difficult to treat, evidence about whether this treatment is more effective in the UK is paramount to decipher whether it should be brought into standard practice.
This trial aims to recruit patients in three different disease groups using a basket trial design enabling three trials to occur in one. The recruitment targets for each disease group are 78 patients for the colorectal group, 66 for the ovarian group and 72 for the stomach group. A delay in opening the trial has reduced the recruitment period to 20 months and it is anticipated that patient recruitment will be challenging. However, patients may drive the motivation to be involved in the trial and hopefully, their local site will be signed up for the trial. With site logistics as a general challenge, there is also a challenge for sites in England to open. This is due to delayed approval for claims of the sites' Excess Treatment Costs (ETCs) process. There is also difficulty opening centres in Scotland and Northern Ireland, therefore, the trial is currently only open in Wales.
Trial Design
All patients will be randomised to receive either standard of care (SOC) systematic anti-cancer therapy (SACT) delivered via the bloodstream/orally or a combination of SOC SACT and/or PIPAC, where three PIPAC procedures are performed. The trial plans to open 30 recruiting/referral sites and 10 PIPAC administering sites with the appropriate training procedures in place for the PIPAC procedure. The trial design enables the variation required to include patients with different cancer types and, the ability to stop the trial for futility in any single cancer type if it is not working whilst continuing with the others.
Efficient communication is key for site set-up, therefore a hub-and-spoke model was adopted for managing common communication within the networks. Patients can be recruited into the trial at either the PIPAC or referral centres, and communication links are important for crucial information transfer for patients randomised into the intervention arm and recruited by a referral centre. We are working to encourage as many sites as possible to become involved in the trial to help reach recruitment targets and also to help the trial access as many patients as possible across the UK.
Involving the Public and Patients
The trial currently has patient representatives for each disease group (colorectal, ovarian and stomach) sitting in the Trial Management Group (TMG), who attend TMG meetings every three months and are given feedback on trial progress. There are also wider Patient Advisory Group (PAG) members with charity-based connections who started attending meetings before the trial commenced and have given feedback on trial design, patient information sheets and feasibility aspects of patient involvement. In a recent PAG meeting, the group were updated on the current stage of the trial. As the trial is only open to recruitment and treating patients in Wales, this meeting gave the members feedback on potential routes to overcome this. Pressure from a patient's perspective driven by using the patient's voice will hopefully influence these processes. The trial will collect valuable information about this type of treatment, and we hope to provide meaningful information from it. The PAG will also be crucial during the latter stages of the trial for dissemination of these results.
Partnerships
The trial team works alongside the International Society for Study of Pleura and Peritoneum (ISSPP). It has strong support from the global PIPAC community as the first RCT in the UK to test the intervention. Support for the trial has been clear in the development of the protocol for the PIPAC procedure and the provision of training on the procedure which is given for free. This is an ISSPP-accredited course that has provided training for many clinical staff involved. The Chief Investigators have also collaborated with industry partners who have provided the equipment required for the procedure at a discounted rate for sites to claim. Alongside several hospitals providing the treatment, there is also a board of experts that forms different committees for the trial. This includes oncologists, surgeons, data analysts, statisticians and patient representatives to give an interdisciplinary perspective on the trial.
The trial team is collaborating with qualitative researchers at the University of Bristol and Swansea University to help establish the impact of this treatment on both patients and the healthcare workers facilitating the treatment. Collaborations have been developed with Radiology experts at Imperial College Healthcare NHS Trust, Cardiff & Vale UHB, Hampshire Hospitals and Royal United Hospitals Bath which have formed a Central Radiology Review Panel. This is because peritoneal tumours are known to be difficult to identify and monitor on scans. This Expert Panel will have oversight by reviewing these scans on a specialised platform called Cimar.
Research Impact
The trial is funded until the end of October 2026 and we will be recruiting until the end of October 2025. Since all patients will be followed up for at least 6 months after they are randomised into the trial, we need to allow time for this and the analysis of the results. With the current trial challenges, the trial team are looking at options on how to boost the recruitment numbers in the trial to achieve meaningful results and offer patients the maximum opportunity to be part of the trial.
The trial results will be the highest level of evidence collected based on UK patients and UK treatment systems. These results can change clinical practice in making PIPAC available to UK NHS patients subject to review by NICE. The results will also be crucial for the design of a confirmatory phase III trial. As well as analysing how patients' tumours are progressing, the trial will collect valuable information on QoL which will also be compared to standard treatment. This could be a large factor in determining the value of patients receiving this treatment as part of SOC.
Follow the Topic
-
ISRCTN: The UK’s Clinical Study Registry

A primary clinical trial registry recognised by WHO and ICMJE that accepts studies involving human subjects or populations with outcome measures assessing effects on human health and well-being, including studies in healthcare, social care, education, workplace safety and economic development.
Your space to connect: The Cancer in understudied populations Hub
A new Communities’ space to connect, collaborate, and explore research on Cancers, Race and Ethnicity Studies and Mortality and Longevity!
Continue reading announcement





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in