The quest for biomarkers in prostate cancer: Multi-sample transcriptomics overcomes heterogeneity and reveals genes with prognostic biomarker potential
Published in Cancer

Prostate cancer is one of the most common cancer types among men worldwide. Although most types of cancer are heterogeneous between individuals, prostate cancer is exceptionally heterogeneous in that most patients have multiple synchronous tumor foci at the time of primary diagnosis. Prostate cancers usually progress relatively slowly and due to the high degree of heterogeneity, patients exhibit very different clinical progression. While some tumors remain indolent for many years, others become malignant and develop metastases rather fast. Current biomarkers for clinical management of prostate cancer are not perfect indicators for patient risk stratification, and it has proven difficult to separate patients with high risk of developing recurrent, aggressive disease from those that do well with only active surveillance. Patient stratification is further complicated by the fact that distinct tumor foci from within the same prostate may possess different aggressiveness and contrasting capacity for disease progression. There is an urgent need for improved diagnostic and prognostic biomarkers that can predict patient outcome and define men at higher risk of developing advanced prostate cancer [1].
Little has been known about the genetic relationship between the different tumor foci that are present within the same prostate gland, and to what degree they are related. We have previously demonstrated that these distinct tumor foci are exceptionally heterogeneous with regard to DNA mutations, and that they only rarely have any somatic mutations in common [2]. In a new paper in Cancer Gene Therapy, we have further assessed inter- and intra-focal heterogeneity also at the transcriptomic level by analyzing multiple tumor samples from each patient [3]. Interestingly, transcription profiles of different malignant foci from within the same prostate showed a similar level of heterogeneity as did tumor samples across patients. The same degree of heterogeneity was found by assessing expressed fusion genes, and expressed somatic mutations. For somatic point mutations and short insertions and deletions, our transcriptome-wide study showed that approximately half of the mutations that had previously been identified on the DNA level were actually expressed. Furthermore, no somatic mutations were found to be expressed across multiple tumor foci from within the same patient.
In essence, the high degree of heterogeneity that we first observed on the genomic level is confirmed also on the transcriptomic level of primary prostate cancer. Based on the heterogeneous nature of this disease, both on the genomic and the transcriptomic level, and also in terms of the highly variable clinical presentation, the distinct tumor foci probably represent tumors of independent origin. Also, the long span of time argues that multiple tumors potentially have the ability to develop through independent evolutionary lineages and thus represent each a separate cancer entity.
Historically, only a single malignant sample from each patient has been included in molecular studies of human cancer, and prostate cancer is no exception. For prostate cancer, however, the associations between certain genetic variants and specific cancer phenotypes have proven less successful, and the fact that each distinct tumor focus within a single prostate gland represents various genomic and transcriptomic potential is a likely explanation. By considering only a single tumor focus from each patient, clinical decisions may even be based on irrelevant or misleading data, if the analysis is not performed on the most relevant focus. Our results have major implications for gene-based testing in future prostate cancer management as it turns out that information from all tumor foci is necessary to draw valid conclusions about each individual’s cancer.
There may, however, be an alternative to molecular testing from all cancer foci. Genes for which the expression levels are similar across distinct tumor foci from within each patient, may have important biomarker potential, if they are also sufficiently variable between patients. Expression profiles of such genes will provide consistent results independent of which malignant tumor focus that is assessed in each individual patient, while also being able to separate the patients into groups. In our research, we have searched specifically for genes with these properties that are also associated with biochemical recurrence while having adequate expression levels across all samples in our cohort. In total, we have identified 16 such genes that we nominated as robust prognostic biomarkers for primary prostate cancer.
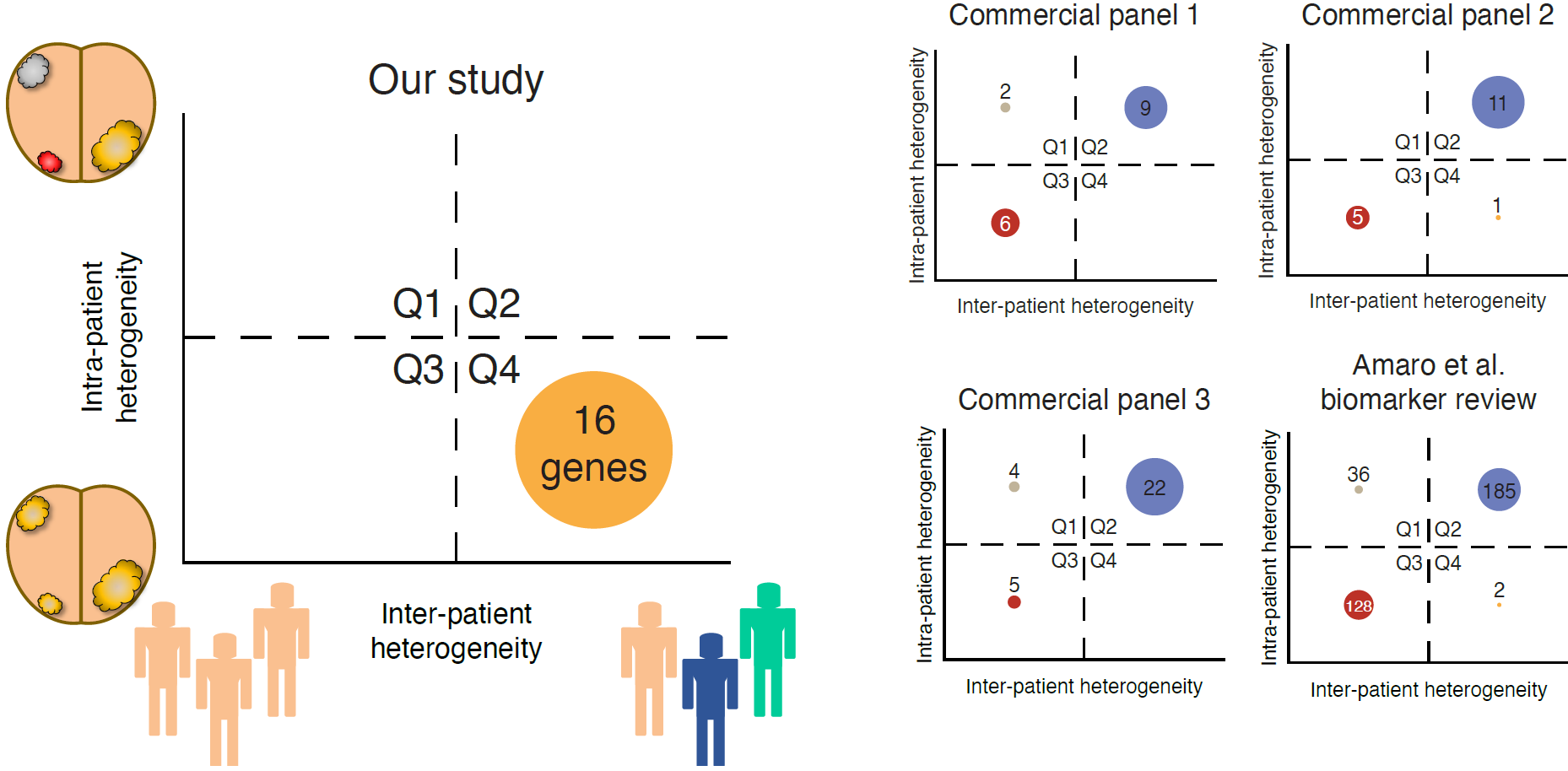
Figure 1. Heterogeneity quadrants for the 16 genes identified in this study, and other selected gene panels. X-axes represent inter-patient heterogeneity, and Y-axes represent intra-patient heterogeneity. Heterogeneity quadrants are derived from coefficients of variation of gene expression values. For each panel, the plot is divided into quadrants by the mean intra-patient heterogeneity and mean inter-patient heterogeneity. The numbers represent the number of genes in each quadrant.
Interestingly, the low variability in expression profiles across different samples from within the same prostate differs from virtually all other proposed prognostic biomarkers for this disease (Figure 1). Gene expression profiling of genes used in three other commercially available prognostication tools, as well as more than 300 genes from a comprehensive review of prostate cancer biomarkers, showed that the expression levels of most of these genes are subject to high intra-patient heterogeneity. This suggests that the prognostic utility of previously identified biomarkers depends largely on which specific focus is being analyzed in each individual patient. Hopefully, our panel of 16 candidate genes will be developed further into a prognostic classifier that can augment current biomarkers in stratification of patients into clinically relevant subgroups in the management of patients with primary prostate cancer.
References
- Kouspou MM, Fong JE, Brew N, Hsiao STF, Davidson SL, Choyke PL, Crispino T, Jain S, et al.: The Movember Prostate Cancer Landscape Analysis: an assessment of unmet research needs. Nature reviews. Urology, 2020. 17(9):499-512.
- Løvf M, Zhao S, Axcrona U, Johannessen B, Bakken AC, Carm KT, Hoff AM, Myklebost O, et al.: Multifocal Primary Prostate Cancer Exhibits High Degree of Genomic Heterogeneity. European Urology, 2019. 75(3):498-505.
- Strømme JM, Johannessen B, Kidd SG, Bogaard M, Carm KT, Zhang X, Sveen A, Mathelier A, et al.: Expressed prognostic biomarkers for primary prostate cancer independent of multifocality and transcriptome heterogeneity. Cancer Gene Therapy, 2022.
Follow the Topic
-
Cancer Gene Therapy

The essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in