The Twist: Losing NEK9 Sparks Pyroptosis — Fiery Cell Death
Published in Cancer

How targeting NEK9 flips cancer cell fate — from survival to explosive death
By Shamima Azma Ansari & Rupesh Dash
🚨 The Problem: When Chemotherapy Stops Working
Chemotherapy often starts strong but hits a wall—tumours adapt, resist, and fight back. For some cancers, resistance is baked in from the start. Should we give up? Or can we outsmart cancer by finding its weakest link?
🔍 Hunting Resistance Genes with a Kinome-Wide Screen
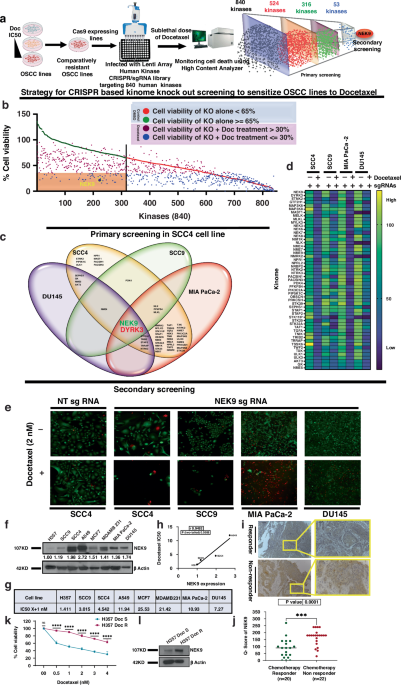
To uncover what’s behind this resistance, we ran a CRISPR/Cas9 screen targeting 840 kinase genes in cancer cells. The goal? Find which genes protect tumours from docetaxel, a common chemotherapy drug.
The standout? NEK9.
🎯 NEK9: From Mitosis Regulator to Chemoresistance Gatekeeper
NEK9 is known for its role in cell division and spindle assembly—but docetaxel resistance? That was new.
Our key findings:
- NEK9 levels are higher in tumours from patients who respond poorly to chemotherapy.
- Knocking out NEK9 made cancer cells dramatically more sensitive to docetaxel.
NEK9 emerged as a molecular switch dictating whether cancer cells live or die during treatment.
💥 The Twist: Losing NEK9 Sparks Pyroptosis — Fiery Cell Death
Deleting NEK9 and treating cells with docetaxel didn’t just increase death—it changed how cells died.
Pyroptosis is a fiery, inflammatory form of cell death. Unlike quiet apoptosis, pyroptosis causes cells to swell, burst, and release signals that rally the immune system.
Why does this matter?
- It flags tumours for immune attack.
- It supercharges tumour destruction.
🧬 The Mechanism: How NEK9 Controls the Wnt Signaling Brake
Here’s the surprise connection: NEK9 keeps a protein called TLE3 in check. TLE3 is a brake on the Wnt signaling pathway, which helps cancer cells survive chemotherapy.
Without NEK9:
- TLE3 floods in, slamming the brakes on Wnt signaling.
- Wnt shutdown strips cancer cells of their survival tools.
- Cells become vulnerable to pyroptosis—a fiery, inflammatory death.
🧩 The Bigger Picture: NEK9, Wnt Signaling, and Tumour Survival
Our data suggest this model:
- NEK9 suppresses TLE3 → Wnt signaling stays ON → cells resist chemotherapy.
- Loss of NEK9 → TLE3 rises → Wnt signaling OFF → pyroptosis triggered → cancer cells die.
🔥 Why Pyroptosis Matters in Cancer Therapy
Most chemotherapies aim to trigger apoptosis. But tumours often block this silent death to survive.
Pyroptosis offers a powerful detour:
- It kills cancer cells explosively.
- It recruits immune cells via inflammation.
- It could turn “cold” tumours into “hot,” more treatable ones.
- It might prevent cancer from coming back.
“It’s not just about killing cancer cells—it’s about how we kill them, and the signals we send to the body.”
💊 A Potential Therapy: Repurposing Fostamatinib to Target NEK9
Could we drug NEK9?
By screening kinase inhibitors, we identified fostamatinib, an FDA-approved drug for immune disorders, as an NEK9 inhibitor.
Combining fostamatinib with docetaxel:
- In cell cultures: Boosted cancer cell death.
- In mouse models: Significantly slowed tumour growth.
💬 Final Thoughts
Our study shows that overcoming resistance isn’t just about killing more cancer cells—it’s about changing how they die.
By removing NEK9’s suppression of pyroptosis, we can give chemotherapy a powerful new edge, making it harder for cancer to survive and hide.
We’re excited about the potential of this approach to create smarter, longer-lasting cancer treatments.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
I loved the way you told this story. Turning it into a kind of scientific saga with superhero vibes made it feel so engaging and accessible. I was just invited to write a Behind the Paper myself, and reading this really inspired me. Thanks so much for sharing!