The Two-Front War Against Cancer and Aging: AI Tools Identify Dual-Purpose Targets for Hepatocellular Carcinoma
Published in Social Sciences, Cancer, and Cell & Molecular Biology
Aging is the dominant risk factor for many chronic diseases, yet it has traditionally been treated as complex “background noise” rather than a target in its own right within pharmaceutical discovery pipelines. This view has been changing, though, with a growing body of evidence suggesting that many of the molecular drivers of late-life diseases are also drivers of aging itself. The implicit lesson, then, is that therapies aimed at these diseases might also modulate aging processes, and vice-versa.
At Insilico Medicine, we develop AI-powered computational tools to exploit the relationships between aging and disease, with the goal of developing drugs to treat both the symptoms and the causes for deeper, lasting therapeutic responses. Our new work in npj Aging leverages our AI toolset to discover new targets for hepatocellular carcinoma (HCC) that show promise in both eliminating cancerous cells and remodeling the disrupted biological networks fundamentally driving aging phenotypes.
The Dual-Purpose Strategy: Linking Drivers of Disease and Aging
The pathological development of aging-related diseases is intertwined with the hallmarks of aging, a set of molecular and physiological phenotypes that take shape throughout the aging process, such as chronic inflammation and cellular senescence. The multifactorial and highly interconnected cellular pathways underlying both aging and aging-related diseases make them difficult to treat, with limited therapeutic options for many conditions. Relatedly, the development and commercialization of therapeutics to treat aging as a general phenotype with the goal of extending healthy longevity is hampered by the difficulty in establishing effective preclinical and clinical models to study effects on aging.
Insilico’s immediate goal to tackle this dilemma is the pragmatic development of drugs for well-defined, aging-related clinical indications where regulatory pathways exist. Our longer-term vision is the more ambitious repurposing of the drugs for other aging-related conditions, and even the aging process itself, once a target is validated and a drug is proven safe and effective in the disease.
Our 2022 landmark study in Aging utilized the company’s AI-based multi-model target discovery platform, PandaOmics, to systematically map the hallmarks of aging to disease-associated genes, identifying 9 "dual-purpose" highly promising candidate targets in an analysis that spanned numerous age-related diseases.
The hallmarks of aging and hallmarks of cancer unsurprisingly contain a number of overlapping dysregulated processes, suggesting that a similar dual-purpose targeting strategy can be effective in the fight against cancer. We identified targets like SIRT1 and GLUD1 in glioblastoma that link metabolic and stress-response pathways to both neuro-oncology and aging biology. Another study expanded this to a massive scale, analyzing omics data across dozens of cancers to find the common denominators of oncogenicity and senescence.
More recently, our work identifying TNIK as a promising target in age-related fibrotic disease due its multifaceted role in regulating diverse signaling pathways converging on dysregulated hallmarks of aging has led to successful clinical trials, showing safety and unprecedented restorative potential for patients with idiopathic pulmonary fibrosis (IPF).
In our newest study, we turn our attention to liver cancer and applying our AI-powered dual-purpose therapeutic discovery approach to a disease with enormous unmet patient need.
Linking HCC to Senescence with AI
Hepatocellular carcinoma (HCC), the most common primary liver cancer, is one of the most lethal forms of cancer globally. As a disease of aging, it often emerges against a background of chronic inflammation, cirrhosis, and cellular senescence. It is this senescence that is both a key pathological driver and a vulnerability that can be a target of therapeutics.
Senescence is a normal process for our cells to undergo, helping restrain tumor growth and aiding in the repair of damaged tissue. However, as we age, senescent cells accumulate because their ability to self-destruct or be cleared by the body diminishes. They become toxic zombies, secreting a cocktail of pro-inflammatory signals and acquiring what is known as the Senescence-Associated Secretory Phenotype (SASP). SASP helps establish a microenvironment in the liver and other organs that promotes the growth of tumors and helps them evade the immune system.
Our study centers on this connection: Can we identify molecular targets that are implicated both in HCC and cellular senescence, and that are suitable for therapeutic development?
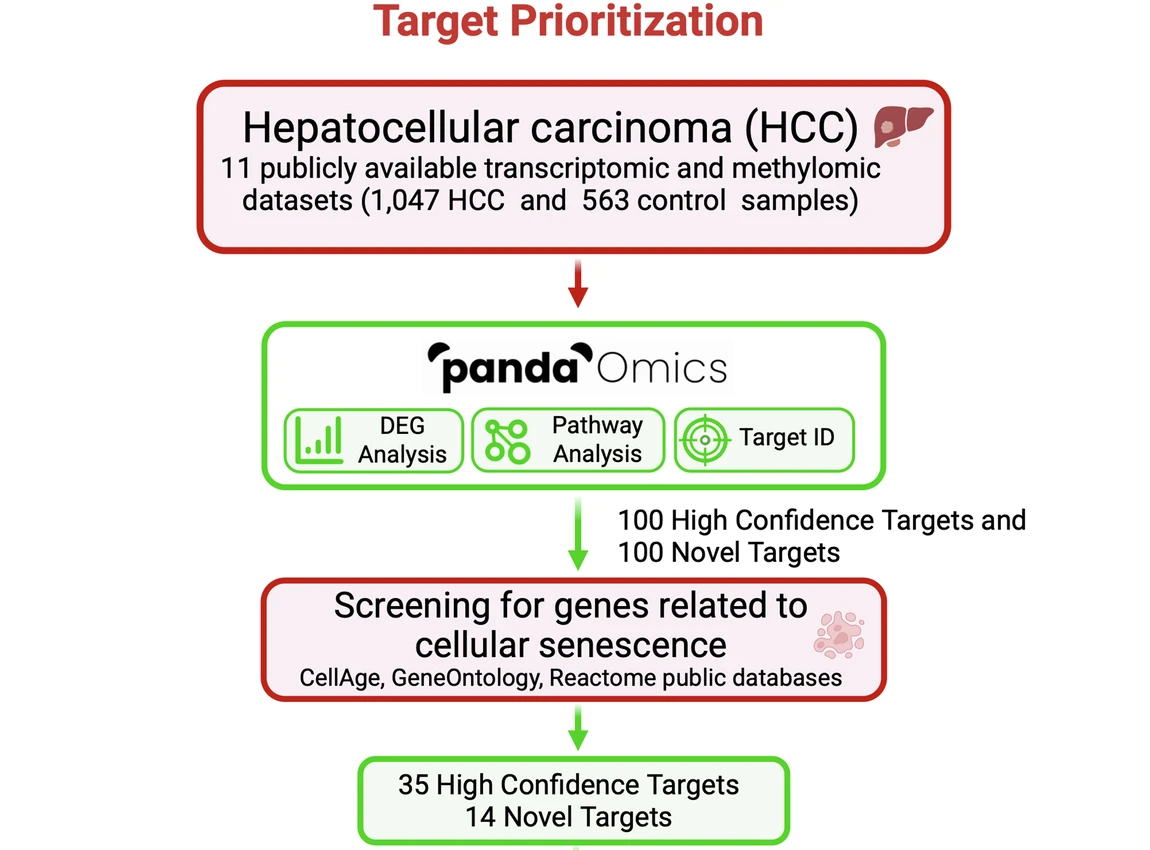
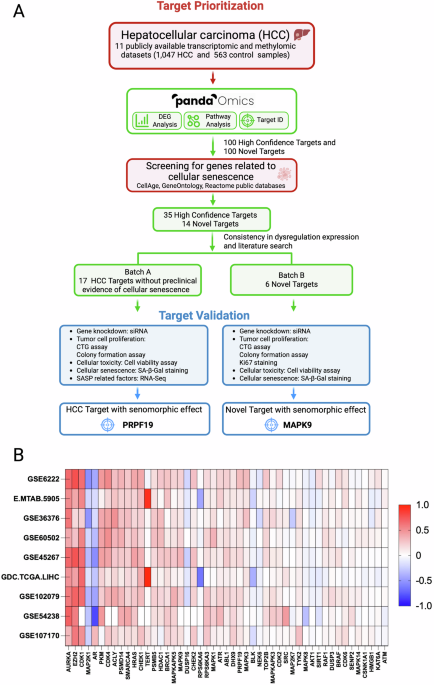
We began with a massive data-mining expedition, perfectly suited for the AI-powered PandaOmics target-discovery platform, which first analyzed 11 large-scale transcriptomic and methylomic datasets, encompassing 1,047 HCC samples and 563 healthy liver samples. The AI looks beyond simple differential gene expression by filtering the results through several lenses, including text mining-based scores derived from scientific research databases to infer gene interaction networks, novelty, druggability, predicted safety, and commercial tractability of any findings.
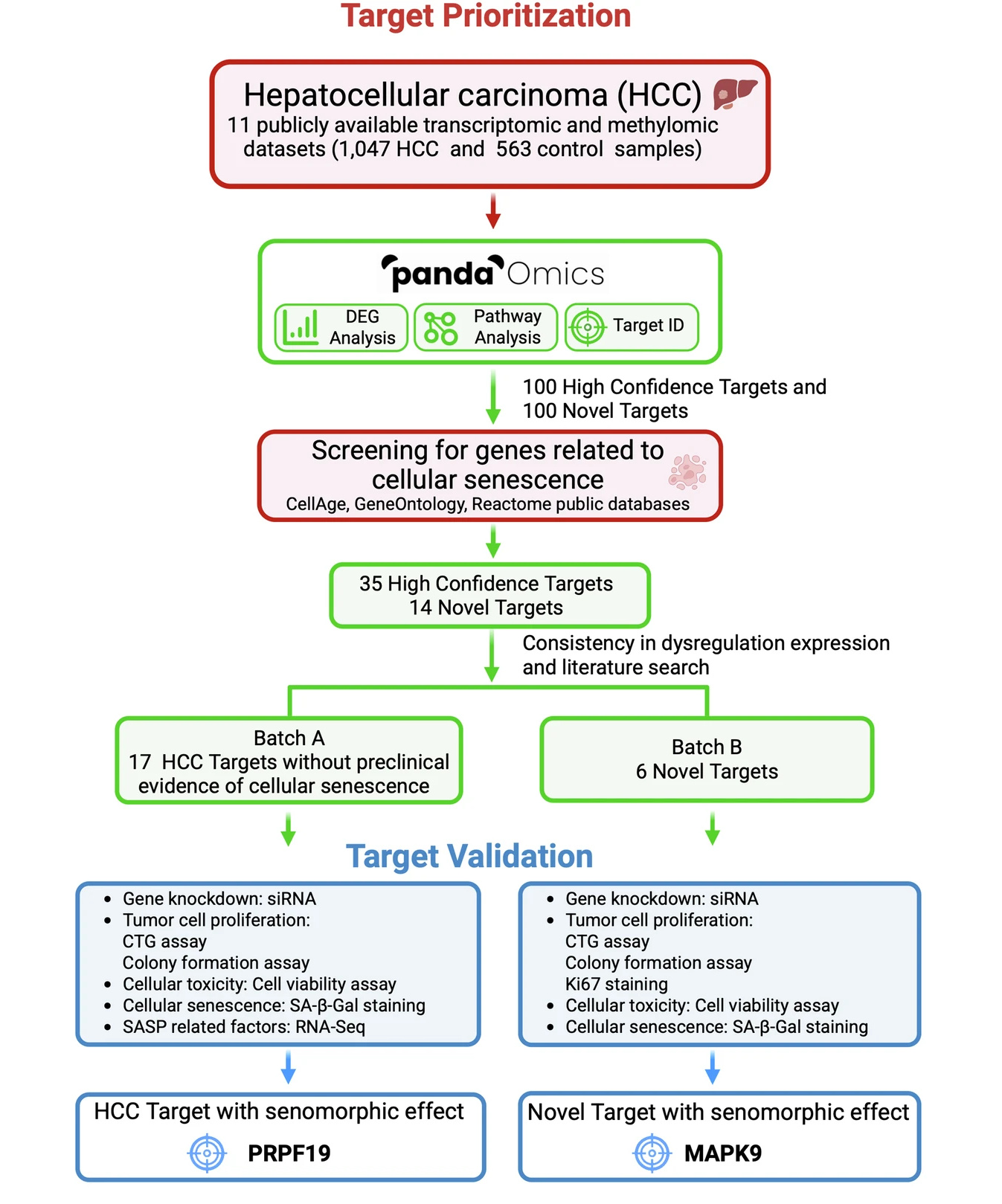
By intersecting the resulting HCC targets with established senescence-associated gene sets (derived from databases like CellAge), we narrowed the field from thousands of candidates to 35 high-confidence dual-purpose targets and 14 targets novelly implicated in senescence.
PRPF19 and MAPK9 as senomorphic targets
Therapeutics that eliminate senescent cells, known as senolytics, have been associated with toxicities related to off-target effects on healthy cells and beneficial senescent cells that limits their therapeutic window. In contrast, senomorphic drugs reverse the senescence phenotype, restoring cell function without killing the cell, potentially offering a safer and more-targeted strategy. An optimal dual-purpose target, therefore, needs to both eliminate cancer cells and reverse the senescence phenotype in senescent cells within the microenvironment.
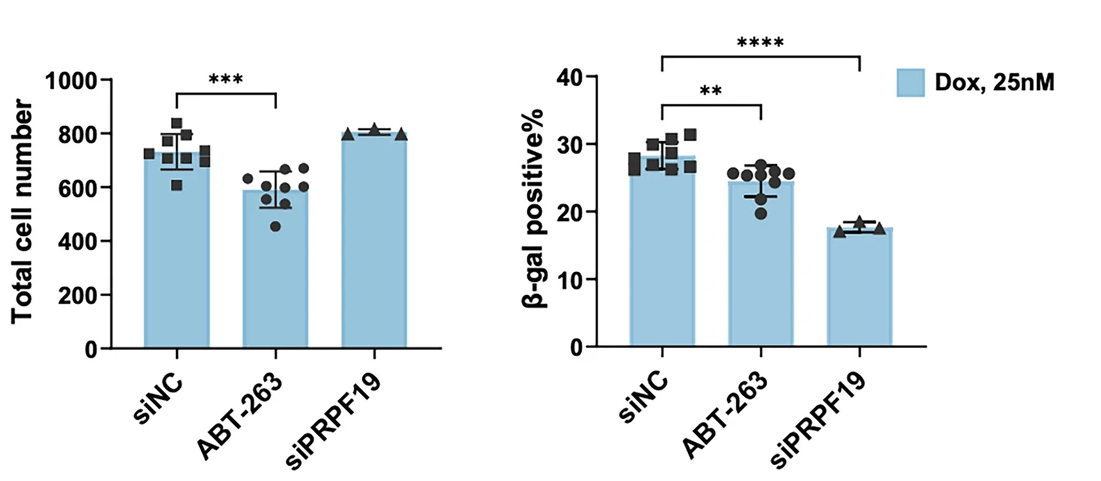
In vitro knockdown of the combined 23 high-priority potential targets further narrowed the list down to the most promising dual-purpose targets. Most of the tested targets reduced the ability of HCC cells to proliferate and form colonies when depleted in vitro, but only two targets could reduce expression of senescence-associated genes while also sparing normal, non-senescent cells: PRPF19 and MAPK9.
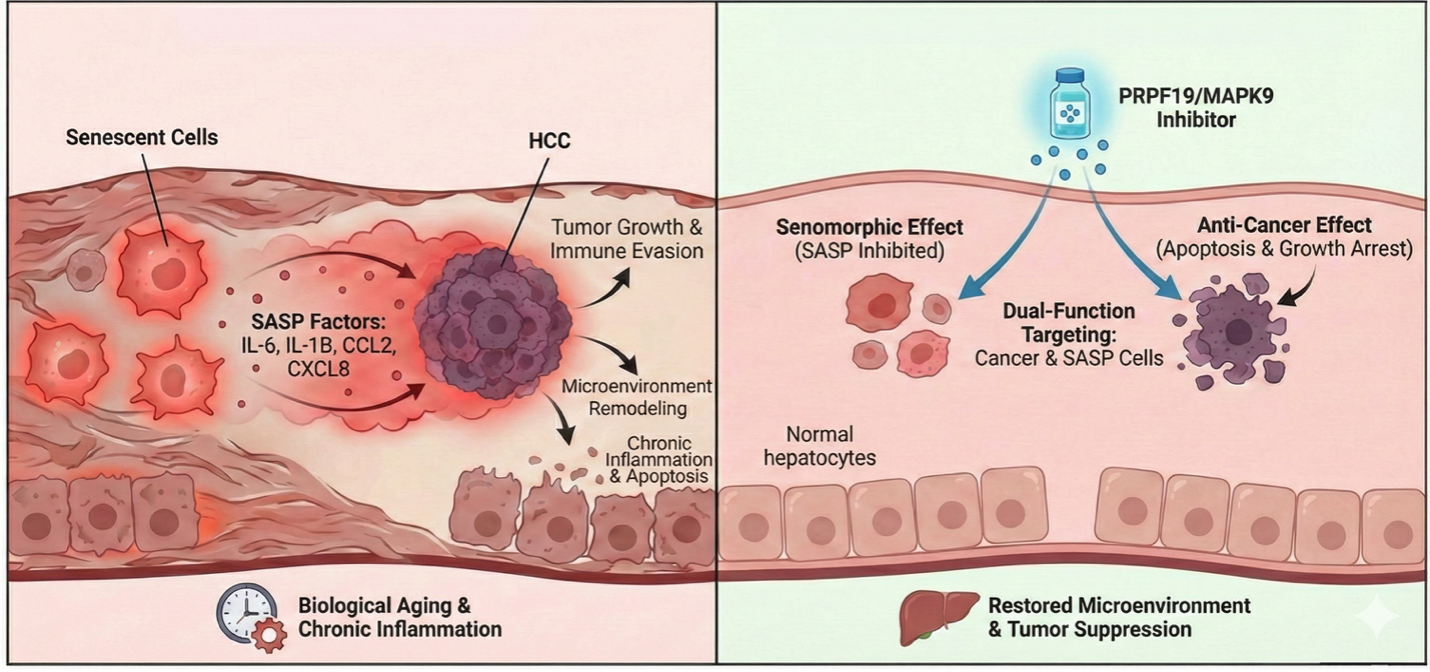
PRPF19 (Pre-mRNA Processing Factor 19) is a component of the spliceosome and is an E3 ubiquitin ligase involved in the DNA damage response. The AI-based analysis revealed that PRPF19 is dysregulated in HCC (which has been shown in previously published studies) but also tightly linked to senescence-associated transcriptional programs. Mechanistically, this makes sense, since efficient DNA repair and accurate RNA processing are central to maintaining cellular homeostasis. With age, dysregulation of these processes contributes to genomic instability and the induction of senescence. In cancer, the same pathways can be hijacked to support uncontrolled proliferation and resistance to stress. PRPF19 may therefore be a central node in both HCC and senescence, where proteostasis, genome maintenance, and cell fate decisions intersect.
MAPK9, also known as JNK2, belongs to the c-Jun N-terminal kinase family, a group of stress-activated kinases with well-established roles in inflammation, apoptosis, metabolism, and aging. MAPK signaling pathways have long been studied in cancer, including HCC, where they regulate proliferation, survival, and response to therapy. At the same time, JNK signaling has been implicated in lifespan regulation and senescence in multiple model organisms. JNK activation has been shown to promote cell death, drive inflammatory senescence-associated secretory phenotypes, or support adaptive stress responses. The AI-driven identification of MAPK9 as a dual-purpose target underscores how deeply conserved stress-response pathways can be leveraged therapeutically. On a more practical level, kinases are among the most druggable classes of proteins, making MAPK9 particularly attractive from a translational perspective.
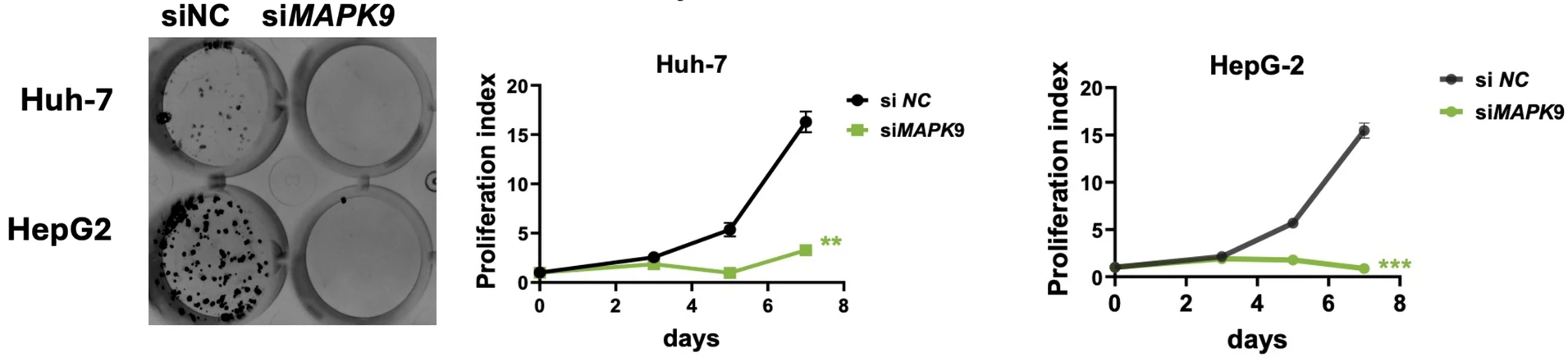
A generalizable strategy, not just two novel targets
The success of this study further validates Insilico’s strategy of developing treatments for aging-related disease by uncovering and disrupting the biological underpinnings that jointly regulate disease and aging. By showing that PRPF19 and MAPK9 are vital for both HCC survival and the maintenance of the senescent state, we refine the roadmap for the discovery of “gero-oncology” drugs. Not only do we lend support to targeting these genes in cancer contexts, but basing the discovery on overlaying multi-omic datasets with aging processes provides a deeper understanding of how they impact both cancerous cells and the tumor microenvironment for greater confidence in their potential as disease targets.
Treating the downstream manifestations of diseases like cancer without simultaneously addressing key upstream drivers may always be a losing game. Developing dual-purpose therapeutics offers an all-encompassing strategy by starting downstream where drugs can be tailored and efficiently studied in the clinic against specific diseases like HCC and gradually extended toward broader modulation of aging processes. The hope, as articulated in this study, is to build a portfolio of well-characterized, clinically validated interventions that can later be repurposed, combined, or adapted for other age-related conditions and aging itself.
As we learn more about the complex, multidimensional phenotypes and regulatory networks of aging, the clearer it is that aging-related diseases are end-stage progression of their dysregulation. Therapeutics that we confidently show can target the disease have the potential to become the most potent weapons in the arsenal against aging. We only need to look at GLP-1 agonist drugs having famously pioneered this approach, from their introduction as diabetes and obesity treatments to their evolving position as the first true longevity drugs. We’re excited to see what other indications these targets can help tackle and the underlying aging processes through which they act.
---
This is the 9th paper Insilico researchers authored in the Nature Publishing Group's journals in 2025, concluding another productive year with significant academic disclosure. In total, Insilico authors contributed to over 50 academic papers including 33 research papers, 13 reviews, and 8 conference abstracts in the year. Other papers published by Insilico authors in 2025 in Nature journals are as follows:
Discovery of a bifunctional PKMYT1-targeting PROTAC empowered by AI-generation
Nature Communications, November 2025
Scientific Reports, October 2025
Modelling sex differences of neurological disorders in vitro
Nature Reviews Bioengineering, October 2025
Nature Medicine, June 2025
A novel, covalent broad-spectrum inhibitor targeting human coronavirus Mpro
Nature Communications, May 2025
Nature Communications, May 2025
Innovations in aging biology: highlights from the ARDD emerging science & technologies workshop
npj Aging, February
Quantum-computing-enhanced algorithm unveils potential KRAS inhibitors
Nature Biotechnology, January 2025
Follow the Topic
-
npj Aging

The mission of this journal is to provide the community with a platform to publish new high-profile insights into all aspects of aging.
Related Collections
With Collections, you can get published faster and increase your visibility.
Sex Differences in Aging Research
Publishing Model: Open Access
Deadline: Sep 21, 2026
Mitochondria at the Heart of Aging: Structure, Function and Failure
Publishing Model: Open Access
Deadline: Apr 10, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in