AI Meets IPF: Taking an AI-Designed Drug from Target Discovery to Phase IIa
Published in Social Sciences, Chemistry, and Computational Sciences
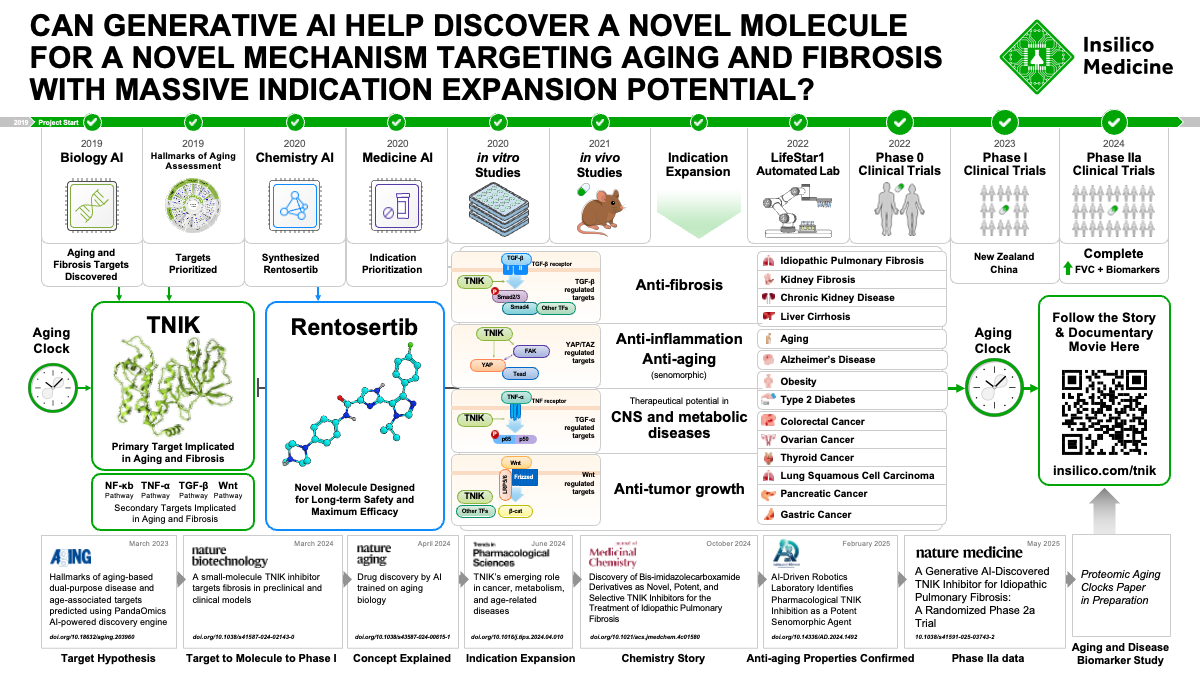
For the first time, a disease target and drug design discovered entirely using artificial intelligence have shown promising results in a Phase IIa trial. At the center of this milestone is rentosertib, a small molecule that could offer new hope to patients with idiopathic pulmonary fibrosis (IPF), marking a leap forward for the field of AI-driven drug development.
A Trial of Firsts
The field of artificial intelligence-based drug discovery is looking for its first big win. Over a decade of concerted development of computational tools for identifying disease-associated molecular targets and designing novel compounds has built enormous excitement and promise for the future of rapid development of highly effective medicines. In fact, at Insilico Medicine, we have now used our suite of AI-based platforms to reduce the average time from project initiation to nominating a clinical candidate molecule to 13 months, far faster than the industry average of 2.5-4 years and for a fraction of the cost. However, none of these advances matter if the drugs can’t prove themselves as safe and effective in gold-standard Phase I-III clinical trials. So far, no drugs coming from AI-powered drug discovery have successfully passed through clinical testing.
We have the chance to change that, though. In this issue of Nature Medicine, we report the positive results from Phase IIa testing of rentosertib (ISM001-055), a small-molecule inhibitor of TRAF2- and NCK-interacting kinase, or TNIK, for the treatment of idiopathic pulmonary fibrosis (IPF).
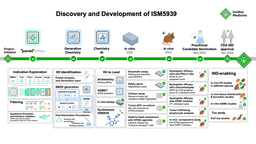
This trial marks several important ‘firsts’ in the realm of AI-powered drug development (AIDD) and for patients suffering from IPF. This is the first clinical trial of a drug designed by AI modulating a disease-associated target identified by AI. TNIK has long been investigated as a target for treating cancer due to its role in regulating Wnt/β-catenin transcriptional target genes, but this is the first therapy modulating TNIK for treating fibrosis. This trial is also the first time a targeted TNIK inhibitor has reached clinical testing. For us at Insilico, this trial is the continuation of our first clinical-stage drug programs entering human testing, demonstrating that our 30-month timeline from project inception to clinical testing may serve as a new benchmark for AIDD workflows.
The success of an AI-discovered compound from discovery to Phase IIa trial is a landmark of the field and of the biotech industry in general. This TINK inhibitor is poised to potentially become the first commercially-available drug that was conceived by AI
——Alán Aspuru-Guzik, PhD, professor of Chemistry and Computer Science at the University of Toronto and director of the Acceleration Consortium
Uncovering the Aging Link in IPF
Idiopathic pulmonary fibrosis (IPF) is an incurable, degenerative, age-related disease leading to the deterioration of lung function and eventually death. Over 3 million people worldwide are affected by IPF, with rates increasing year-over-year as the global population ages and experiences higher incidence of age-related diseases. The lineup of approved, standard-of-care treatments for IPF centers on pirfenidone and nintedanib, drugs targeting the pro-fibrotic and pro-inflammatory processes implicated in IPF pathology. These drugs, approved for use in 2014, slow the deterioration in lung function for up to 24 months, as measured by forced vital capacity (FVC), but neither leads to a gain in FVC or is curative1,2. Novel therapies are desperately needed to address this increasingly dire unmet need to effectively treat IPF.
As an age-related disease, with people aged 75 years and older being diagnosed over 60 times more frequently than people aged 18-343, treatments for IPF have the potential to be dual-function therapeutics, or drugs that treat both aging and age-related disease. The aging process is characterized by accumulating dysregulation of various metabolic, immune, and homeostatic pathways across tissues, and much of the research into combatting aging is focused on modulating key genes within these pathways. At Insilico Medicine, we leverage advanced artificial intelligence (AI) models trained on multi-omic datasets, publication and grant text-mining, and other sources of biological insights to find new targets for aging-related diseases.
As we previously described alongside publication of our preclinical work published in Nature Biotechnology4, we aimed our AI-powered Pharma.ai drug discovery platform at IPF. Our target discovery engine PandaOmics identified TRAF2- and NCK-interacting kinase (TNIK) as the top target, a novel finding for IPF.
Drug development in IPF has proved to be challenging with multiple late phase trial failures in the last decade. Insilico’s exciting AI-based identification of a TINK inhibitor as a potential treatment for IPF provides an important roadmap for the rationale identification of therapeutic targets for pulmonary fibrosis and the development of drugs that stand a much higher chance of achieving approval for this life-shortening disease.
——Toby Maher, MBBS, MSc, PhD , Professor of Clinical Medicine, Director of Interstitial Lung Disease, Keck School of Medicine of the University of Southern California
TNIK’s Emerging Role in Age-Related Disease
We have found through our analyses integrating aging clocks into our target discovery engine that TNIK is a central hub gene implicated in biological aging. Aging clocks have been developed by different research groups to identify the specific biological processes that become progressively dysregulated with age. Different aging clocks rely on different input data, such as gene expression profile or DNA methylation profile, to predict age from an individual’s biosample. TNIK consistently emerges as a highly predictive gene and epigenetic locus across different published aging clocks, supporting its investigation as a target for aging-related disease (Figure 1).
Inspiring decades of pharmaceutical pursuit, TNIK has been found to be involved in activation of targets of Wnt/β-catenin signaling5, a key pathway controlling differentiation and proliferation that is frequently dysregulated in a variety of cancers. TNIK has therefore been extensively investigated preclinically and clinically as a target for anticancer therapeutics. TNIK has also been found to regulate diverse cellular signaling pathways, such as TGF-β, NF-kB, and Hippo, which, unsurprisingly, implicates TNIK as an important mediator of a variety of disorders heavily associated with the hallmarks of aging6. Researchers have linked TNIK, in addition to cancer, to glucose metabolism and metabolic disorders like obesity7, neurological disorders like Alzheimer’s disease8–10, and, in our own work, to fibrosis4. Through the lens of the individual hallmarks of aging, TNIK has been associated with chronic inflammation, cellular senescence, deregulated nutrient sensing, genomic instability, stem cell exhaustion, and altered intercellular communication11. On a more pragmatic level, such diverse roles of TNIK across physiological process implicated in disease position it as an ideal target to pursue indication expansion once the safety of a TNIK-targeted therapeutic is established in clinical testing (Figure 1).
The GENESIS of a New IPF Therapy
Based on our promising preclinical findings using our novel TNIK inhibitor, rentosertib (ISM001-055), to treat in vitro and in vivo models of pulmonary, kidney, and skin fibrosis, we launched a series of Phase 0/I clinical studies testing rentosertib treatment in healthy individuals. Based in Australia, New Zealand, and China, these studies found rentosertib to be safe in healthy individuals with no significant drug accumulation or drug metabolism enzyme red flags, which encouraged us to continue testing rentosertib in further studies in patients (Figure 1).
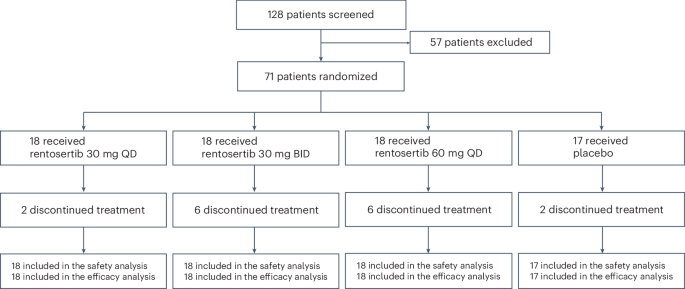
In this issue of Nature Medicine, we report the results from the Phase IIa GENESIS-IPF trial (Generative AI Enabled Novel Experimental Study of ISM001-055 in Subjects with Idiopathic Pulmonary Fibrosis), a multicenter, double-blind, randomized, placebo-controlled trial testing the safety and efficacy of rentosertib administered over 12 weeks in a cohort of 71 enrolled patients with IPF in China (NCT05938920). Patients were administered 30 mg once-daily (QD, n=18), 30 mg twice-daily (BID, n=18), or 60 mg QD rentosertib (n=18), or placebo (n=17) and monitored for the occurrence of adverse events and for changes in lung function (Figure 2).
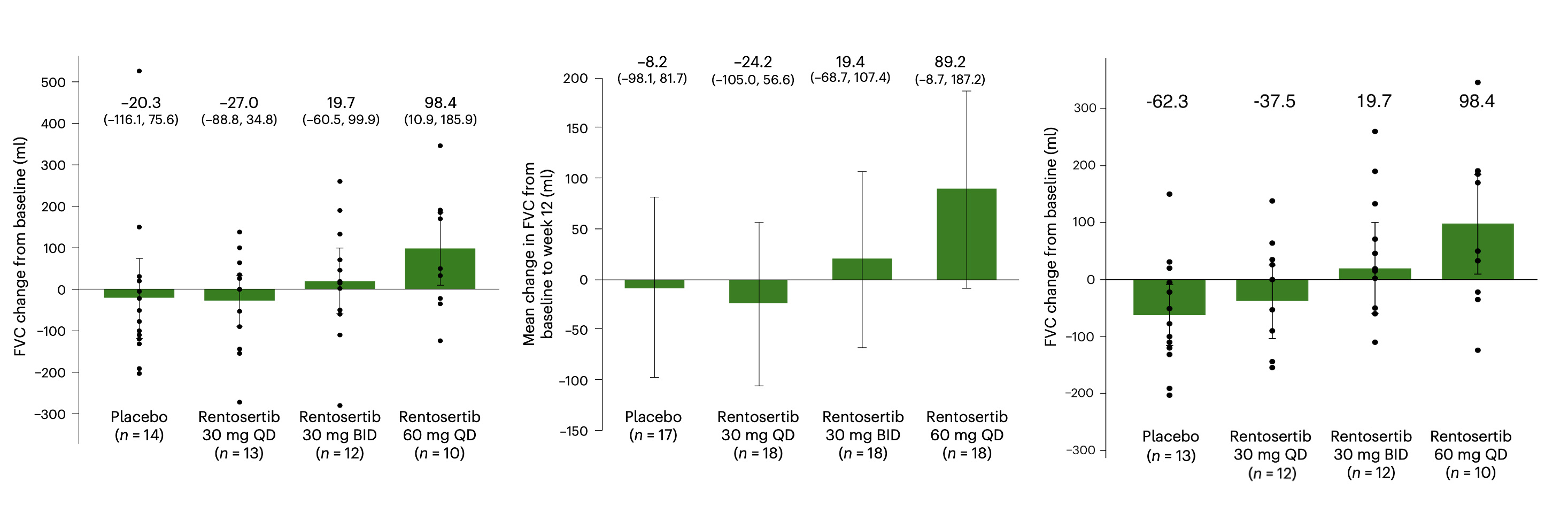
The study was conducted across 22 unique sites, with all patients enrolled within nine months starting in June 2023, and with the final patient completing study in August 2024, marking this as an exceptionally collaborative study with wide interest and participation across the interstitial lung disease community in China. Based on imaging and clinical data of the study population, we believe the trial participants are similar to and representative of global IPF patient cohorts, such as the PROFILE cohort in the UK12. We are therefore optimistic that the study’s results can be applied broadly across IPF patients worldwide.
The study found a manageable safety profile, with similar rates of adverse events (AEs) across all treatment arms and a low rate of serious AEs (grade 3+, i.e., life-threatening or required hospitalization) among all treatment arms, while AEs resolved upon cessation of drug administration.
Perhaps most encouragingly, patients receiving 60 mg QD rentosertib experienced an increase in forced vital capacity (FVC), the gold-standard metric assessing lung function in IPF patients, following 12 weeks of treatment (+98.4 mL [95% CI, 10.9 to 185.9]), whereas patients receiving placebo experienced a mean decrease in FVC (-20.3 mL [95% CI, -116.1 to 75.6]), reflective of the inability of current standard-of-care treatment regimens to consistently restore lung function. Breaking down the cohort by whether patients were concurrently taking standard-of-care drugs, either nintedanib or pirfenidone, which was approximately half the cohort, we found that patients experienced a similar trend of response, with those receiving 60 mg QD rentosertib having an average improvement in FVC and those receiving placebo having an average decrease in FVC. The low number of patients in each group after this sub-grouping, however, require further follow-up and prospective testing in larger cohorts, which we are planning in upcoming trials.
As an exploratory endpoint, patient serum was collected throughout the trial and was profiled for circulating proteins to investigate mechanism of action and prognostic or predictive biomarkers of response to rentosertib treatment. We uncovered modulation of fibrosis- and inflammation-associated proteins, finding dose- and time-dependent changes in serum proteins and lung function after 12 weeks of treatment correlated with anti-fibrotic and anti-inflammatory effects of rentosertib. For example, expression of COL1A1, a gene involved in producing collagen and often elevated in fibrosis, decreased over the 12-week course of treatment in all rentosertib treatment arms but most significantly in those receiving 60 mg QD rentosertib. Not only that, but the change in COL1A1 expression from baseline to end-of-treatment (week 12) inversely correlated with the change in FVC patients experienced (Figure 3). This points to COL1A1 as a potential marker to predict which patients may respond best to rentosertib treatment and that extracellular matrix deposition and remodeling, hallmarks of fibrosis, was reduced or even reversed in responding patients. Similarly, the expression of IL10, an anti-inflammatory cytokine, increased over time with 60 mg rentosertib treatment, and the change in its expression correlated with change in FVC across patients (Figure 3).
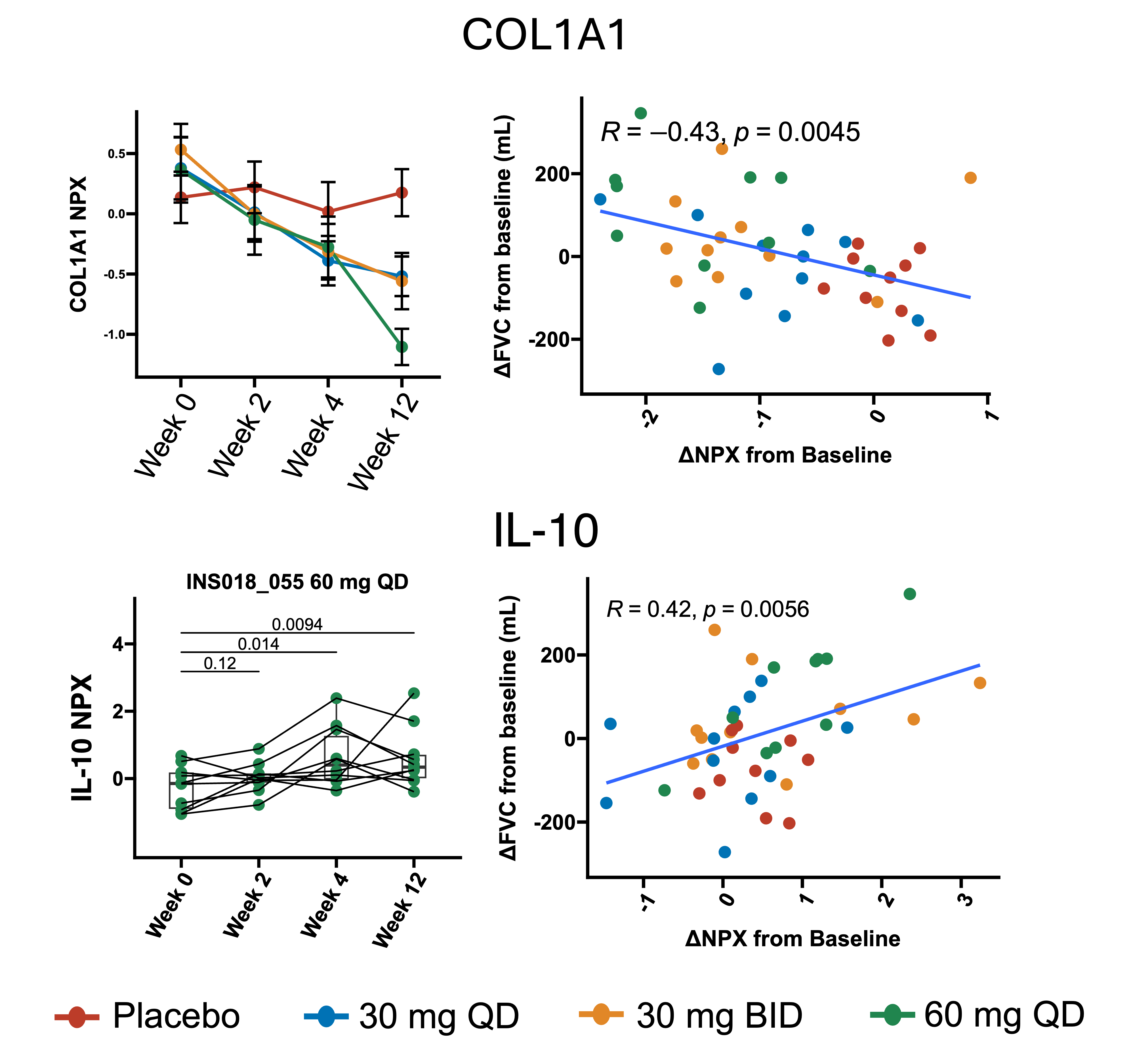
.
The Phase 2a trial results for Rentosertib are promising, showing a manageable safety profile with early signs of improved lung function in patients with idiopathic pulmonary fibrosis. The observed increase in forced vital capacity at the 60 mg dose, along with consistent pharmacokinetics and downregulation of key profibrotic biomarkers such as COL1A1 and MMP10, suggest that TNIK inhibition could be a novel and meaningful approach in this devastating disease. The publication of these findings in Nature Medicine underscores the credibility and potential clinical relevance of this first-in-class, AI-discovered therapy - marking a significant step forward in the pursuit of precision treatment for fibrotic lung disease.
——Tejaswini Kulkarni, MD, MPH, Associate Prof, Pulmonary, Allergy and Critical Care Medicine, Director, Interstitial Lung Disease Program, Associate Medical Director, Lung Health Center, The University of Alabama at Birmingham
Pioneering the Future of IPF and Age-Related Disease
A parallel Phase IIa trial being conducted in the US is currently enrolling patients (NCT05975983), with a similar trial design scheme. We hope to validate these findings in this ongoing Phase IIa and in planned subsequent trials of longer duration and larger cohort size. The ability to reverse the deterioration of lung function in patients with IPF would truly be a groundbreaking advance for this growing population with tremendous unmet medical need. It’s been a privilege to contribute to a potential turning point in how IPF is treated, bringing novel drugs to those affected by IPF and other age-related disease using advanced AI tools. This may mark not just a milestone for IPF treatment, but a glimpse into the future of how we discover, test, and deliver therapies for a wide range of diseases.
References
- Richeldi, L. et al. Efficacy and Safety of Nintedanib in Idiopathic Pulmonary Fibrosis. New England Journal of Medicine 370, 2071–2082 (2014).
- King, T. E. et al. A Phase 3 Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. New England Journal of Medicine 370, 2083–2092 (2014).
- Raghu, G., Weycker, D., Edelsberg, J., Bradford, W. Z. & Oster, G. Incidence and Prevalence of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 174, 810–816 (2006).
- Ren, F. et al. A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models. Nat Biotechnol 1–13 (2024) doi:10.1038/s41587-024-02143-0.
- Mahmoudi, T. et al. The kinase TNIK is an essential activator of Wnt target genes. The EMBO Journal 28, 3329–3340 (2009).
- López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: An expanding universe. Cell 186, 243–278 (2023).
- Pham, T. C. P. et al. TNIK is a conserved regulator of glucose and lipid metabolism in obesity. Science Advances 9, eadf7119 (2023).
- Anazi, S. et al. A null mutation in TNIK defines a novel locus for intellectual disability. Hum Genet 135, 773–778 (2016).
- Abreha, M. H. et al. TBK1 interacts with tau and enhances neurodegeneration in tauopathy. Journal of Biological Chemistry 296, (2021).
- Jiang, J. et al. Mutations in the postsynaptic density signaling hub TNIK disrupt PSD signaling in human models of neurodevelopmental disorders. Front. Mol. Neurosci. 17, (2024).
- Pun, F. W. et al. Hallmarks of aging-based dual-purpose disease and age-associated targets predicted using PandaOmics AI-powered discovery engine. Aging 14, 2475–2506 (2022).
- Maher, T. M. et al. An epithelial biomarker signature for idiopathic pulmonary fibrosis: an analysis from the multicentre PROFILE cohort study. The Lancet Respiratory Medicine 5, 946–955 (2017).
Follow the Topic
-
Nature Medicine

This journal encompasses original research ranging from new concepts in human biology and disease pathogenesis to new therapeutic modalities and drug development, to all phases of clinical work, as well as innovative technologies aimed at improving human health.
Your space to connect: The Primary immunodeficiency disorders Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine, Immunology, and Diseases!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Stem cell-derived therapies
Publishing Model: Hybrid
Deadline: Mar 26, 2026
Digital Medicine for Infectious Diseases
Publishing Model: Hybrid
Deadline: Nov 09, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in