Harnessing AI to Transform STING Modulation in Cancer Immunotherapy
Published in Cancer, Chemistry, and Computational Sciences
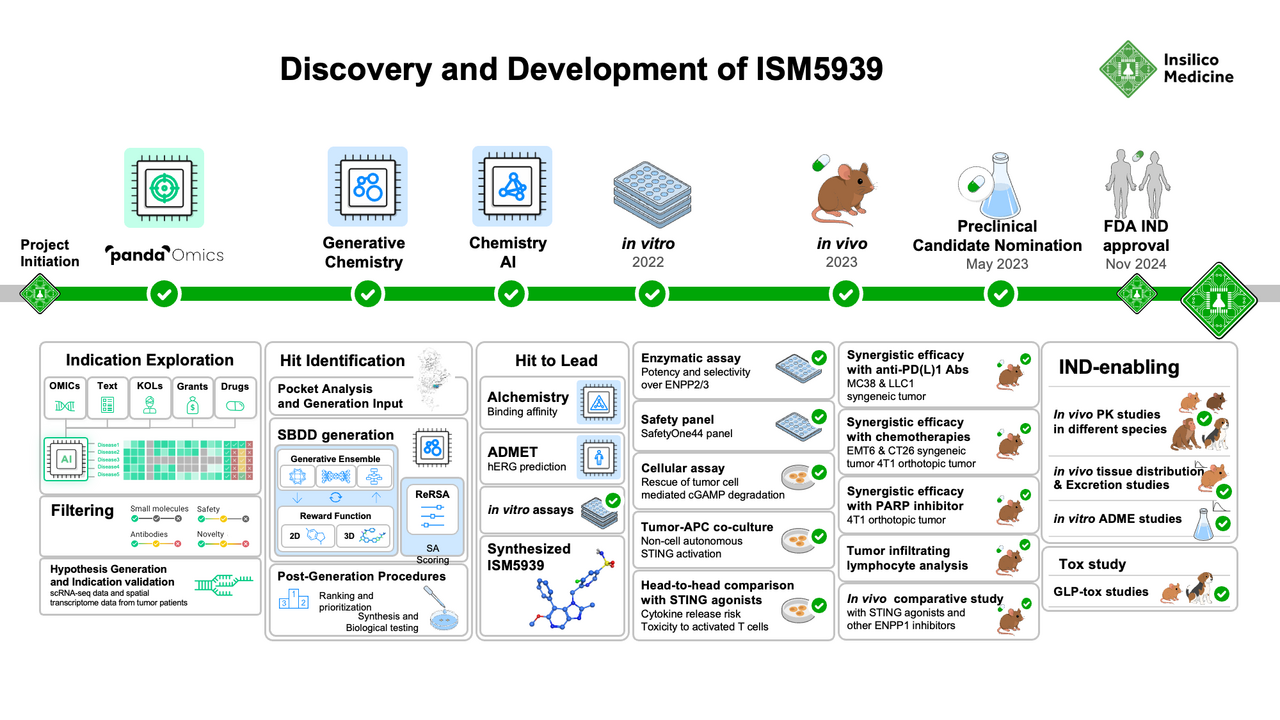
Insilico Medicine has pioneered the development and implementation of artificial intelligence (AI)-based tools for drug discovery, from target identification to indication prioritization, from generating novel compound structures to predicting ADMET properties, and from clinical trial design and prediction to generative AI research assistance. Now published in Nature Communications, our team shares our work characterizing ENPP1 as an inhibitory immune checkpoint across solid tumors, using AI tools to design a novel ENPP1 inhibitor, and validating ENPP1 inhibition in preclinical models as a promising immunotherapy strategy to activate the cGAS-STING pathway safely and effectively.
A Fresh Look at the STING Axis
The cGAS-STING pathway has long held interest as an endogenous, potent activator of downstream pro-inflammatory signaling that can be harnessed for antitumor immunotherapy. The dampening of cGAS-STING signaling in the immunosuppressive tumor microenvironment (TME) suggests that its restoration or amplification may promote anti-tumor immunity by STING-responsive immune cells in the TME.
The cGAS enzyme acts as a powerful sensor of cytosolic DNA, a common feature of cancerous cells, initiating cytokine signals to activate the STING (stimulator of interferon genes) protein in nearby antigen-presenting cells (APCs) and triggering type I interferon responses and cascading proinflammatory signals (Figure 1). Yet, despite the promise of cGAS-STING pathway activation in cancer immunotherapy, translating this biology into safe and effective treatments has remained a formidable challenge. Direct STING agonists have struggled with poor pharmacokinetics and safety liabilities, often inducing systemic cytokine storms, reducing the endogenous adaptive immune response, or failing to produce durable responses.
This left us with a simple question: Is there a safer, smarter way to activate STING in tumors?
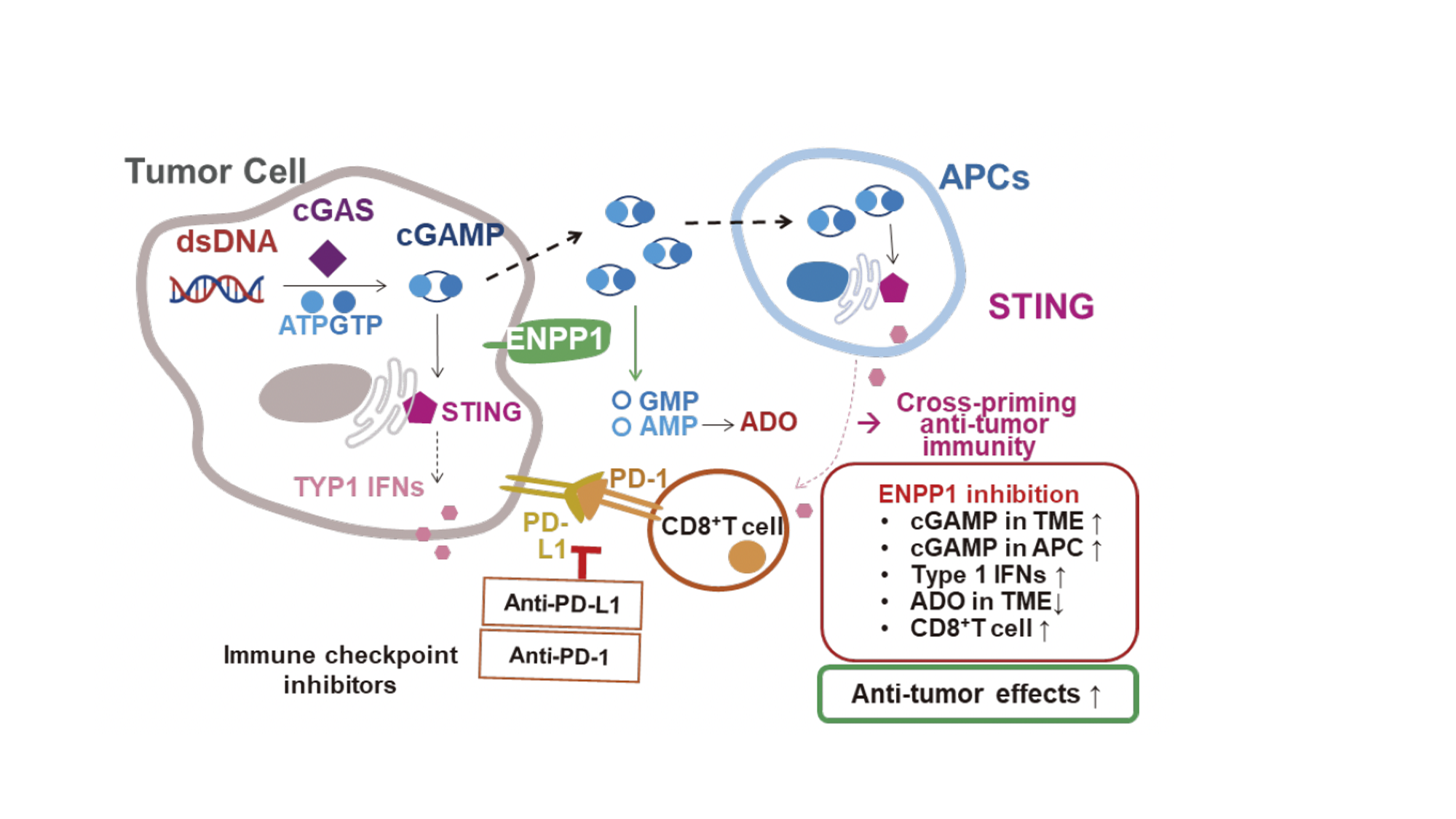
Figure 1. The cGAS-STING signaling pathway. Double-stranded DNA sensor cGAS initiates a signaling cascade by catalyzing second-messenger cGAMP, which induces STING activation in TME-resident APCs, leading to strengthened anti-tumor immune responses. ENPP1 degrades cGAMP into constituent nucleotides GMP and AMP, reducing effective cGAMP levels in the TME. Further dephosphorylation of AMP results in Ado, whose accumulation promotes an anti-inflammatory microenvironment. See Borja Ruiz-Fernández de Córdoba, et al. Clin Cancer Res.2023;29(12):2184-2193.
We turned our attention to a natural antagonist of STING activation, ENPP1, an enzyme that degrades extracellular cGAMP, the key second messenger synthesized by cGAS that activates STING. ENPP1 is known to be particularly highly expressed in the tumor bed of solid cancers. We reasoned that boosting cGAMP generated within the tumor itself by preventing its degradation may be a promising strategy for activating STING without the complications of direct, systemic STING activation. Inhibiting ENPP1, importantly, would allow for a more subtle approach by letting cGAMP accumulate locally, activate STING in APCs, and carry out its antitumor effects, such as licensing APCs, upregulating co-stimulatory molecules on APCs, and dampening myeloid-derived suppressor cell activation, T-cell suppression, and epithelial-mesenchymal transition of tumor cells, as well as potentially toxic effects such as massive cytokine release, specifically within the TME. In essence, we aimed to unlock STING from within the tumor, not force it open from the outside.
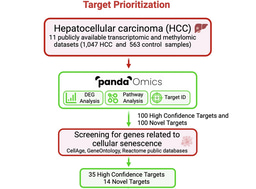
The first step was to identify the conditions where ENPP1 inhibition could actually work. ENPP1 is known to be involved in cancer onset, progression, and metastasis, and evidence supports its role as a suppressive immune checkpoint in triple-negative breast cancer (TNBC). Whether ENPP1 acts as an immune checkpoint across a broad range of solid tumors, however, was unknown.
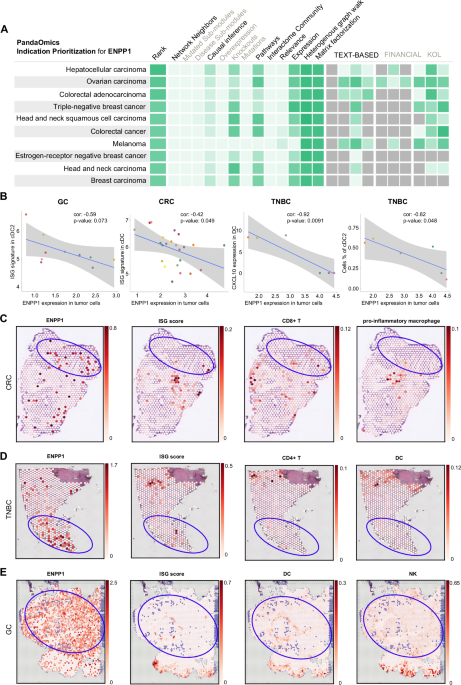
For this, we turned to our AI-powered target discovery platform, PandaOmics, which synthesizes massive multi-omics datasets, text mining-derived genetic interactions from publications, grants, and patents, and other sources of biological interaction network information to identify high-potential targets in disease (Figure 2). We used the indication prioritization tools within PandaOmics to ask which indications ENPP1 inhibition would be most likely to be efficacious against. TNBC and other cancer types, such as hepatocellular carcinoma, gastric cancer, and colorectal cancers, emerged as the top candidate indications. Validating our predictions with gene expression data from The Cancer Genome Atlas (TCGA) and spatial transcriptomic analyses, which showed that these cancer types consistently highly express immunosuppressive cGAS and ENPP1 and depend on ENPP1 for prognosis-associated STING-mediated interferon signaling, gave us confidence that ENPP1 acts as a targetable central node in immunosuppressive signaling across a range of solid tumors.
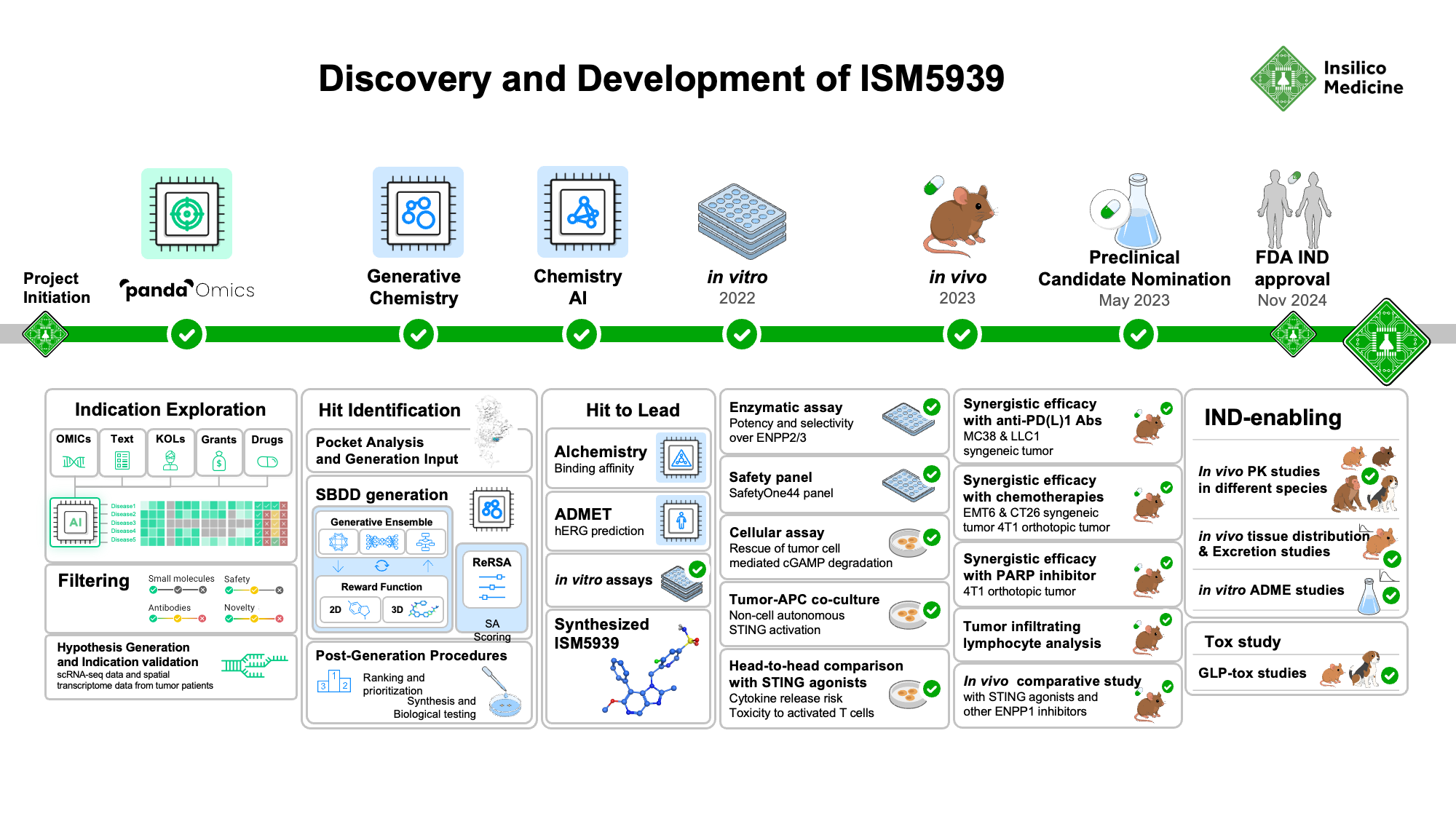
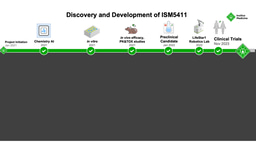
Figure 2. Discovery and development timeline of novel ENPP1 inhibitor, ISM5939. Insilico Medicine’s AI-powered indication prioritization platform identified oncology indications most likely to respond to ENPP1 inhibition. Generative chemistry AI tools and molecular optimizations led to design of ISM5939, which was extensively tested in vitro and in vivo as a mono- and combination therapy with checkpoint inhibitors and DNA-damaging chemotherapy agents against various solid tumor models.
Smart Chemistry, Intelligent Design
Having identified ENPP1 as a promising immunomodulatory target, we next faced the challenge of pharmacologically inhibiting it effectively. ENPP1 itself has proven to be an elusive target. Existing inhibitors often suffer from poor potency at physiological pH, limited selectivity, and unfriendly pharmacokinetics. The challenges associated with traditional drug discovery, such as time and cost of screening for effective inhibitory compounds and the interplay with complex biological pathways, are magnified. We knew we needed not only better chemistry, but a smarter way to discover and optimize molecules that addresses the factors that have so far prevented STING modulators from succeeding in the clinic.
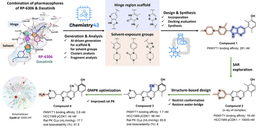
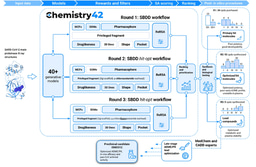
We turned to Chemistry42, our generative chemistry platform, which uses deep generative models and reinforcement learning algorithms to design compounds tailored for specific binding pockets. Using known ENPP1 inhibitors as a starting point, the platform iteratively generated novel chemical structures, evaluating each against a multi-parametric optimization framework. This included not only binding affinity, but also synthetic accessibility, novelty, ADMET properties, and the potential for off-target effects.
From this AI-guided exploration, we rapidly identified candidate inhibitors, starting with an initial hit compound over 50 times more potent than the known starting-point compound (IC50 2.55 nM vs 142 nM). Subsequent systematic optimizations to key functional domains to improve predicted binding free energy, stability, and pharmacokinetics, abate CYP3A4 liver enzyme induction to prevent drug-drug interactions, and curtail hERG cardiac potassium channel inhibition to avoid cardiotoxicity led to lead compound ISM5939.
In total, we were able to generate novel hit molecules in under three months from initiation using Chemistry42’s design and property prediction capabilities.
Safer, Contextual STING Activation
ISM5939 exhibited potent ENPP1 inhibition against both in vitro and in vivo mouse and human tumor models, showing low-nanomolar IC50 and enhanced accumulation of tumor-secreted cGAMP across diverse cancer types, including breast, renal, colorectal, melanoma, and lung cancers. The dose-dependent cGAMP accumulation was ablated in ENPP1-deficient tumor models and when ISM5939 treatment accompanied inhibitors of STING or downstream effector cytokine IRF3, highlighting the STING-dependent mechanism of ISM5939-induced cGAMP accumulation.
ISM5939 had excellent oral bioavailability, unlike many legacy inhibitors with suboptimal activity at physiological pH or low selectivity. ISM5939 maintained high potency in acidic tumor-like environments and showed remarkable specificity for ENPP1 over related enzymes like ENPP2 and ENPP3 as well as a panel of clinically relevant potential off-targets.
Perhaps most encouragingly, ISM5939 did not induce the massive cytokine release or STING-induced circulating lymphocyte depletion characteristic of direct STING agonists in vitro or in vivo, even when administered at high concentrations, giving it a dramatically wider therapeutic index. This feature may make ISM5939 particularly valuable in combination regimens, especially in immunologically "cold" tumors where conventional immunotherapy fails. In vitro and in vivo studies across multiple tumor models revealed that ISM5939 synergizes powerfully with both immune checkpoint inhibitors and DNA-damaging chemotherapies, inducing a more immunologically active and cytotoxic TME, even in tumors resistant to anti-PD-1/PD-L1 monotherapy.
We also began to identify biomarkers that predict response to ISM5939, like LRRC8A, a cGAMP transporter. We found that within LRRC8A-high tumor samples, PD-1/PD-L1-resistant tumors had high ENPP1 expression, suggesting LRRC8A and ENPP1 expression together may predict patients for whom combination immunotherapy can overcome resistance. Similarly, ENPP1 expression was found to be higher in patient tumors resistant to chemotherapy, and cGAS-low/ENPP1-low cancer models showed a lower response to ISM5939, further suggesting ENPP1 and genes of the cGAS-STING pathway may be biomarkers of response and could guide patient selection in future trials testing ENPP1 inhibition for treating solid tumors.
Where We Go Next
This study marks the first AI-designed ENPP1 inhibitor to enter preclinical development, and we are now preparing for a Phase I clinical trial with recent FDA approval of our dose design as an investigational new drug (IND: 172399). While challenges remain, such as understanding resistance mechanisms and refining predictive biomarkers, ISM5939 represents a new generation of STING modulators: localized, tumor-selective, and intelligently designed.
Not only did our AI-powered drug discovery yield a potentially first-in-class ENPP1 inhibitor for treating a variety of solid tumors, but it accelerated the timeline, showcasing what is possible through generative AI platforms for the benefit of patients in need of novel therapy options. What used to take years of wet-lab iteration was compressed into months. In fact, our average time from program initiation to candidate compound nomination now takes 13 months, far lower than the multi-year traditional drug development pipeline, allowing drugs like ISM5939 to reach clinical testing and patients much sooner.
Looking back, this journey was about rethinking what’s possible in immunotherapy by bringing together biology, computational science, and a belief that smarter design leads to safer treatments. We hope ISM5939 inspires further work into ENPP1 inhibition and sparks a new generation of drugs that can finally unlock the potential of STING modulation with the precision of AI-based platforms and the potency to meet today’s demand for novel and improved therapies for patients in need.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Ask the Editor - Immunology, Pathogenesis, Inflammation and Innate Immunity
Got a question for the editor about the complement system in health and disease? Ask it here!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in