TRAIP suppresses bladder cancer progression by catalyzing K48-linked polyubiquitination of MYC
Published in Cancer
Bladder cancer (BLCA) poses a significant global health burden, with approximately 570,000 new cases and 210,000 annual fatalities [1]. Deciphering the molecular intricacies governing BLCA progression is imperative for advancing diagnostic and therapeutic interventions. TRAIP, initially identified as a TRAF1 and TRAF2 interactor, plays a crucial role in genome stability through its E3 ubiquitin ligase activity [2, 3]. While recent studies underscore TRAIP’s involvement in various cancers, its specific impact on BLCA and its consistent role in tumorigenesis remain elusive. Notably, TRAIP’s counterpart, TRAF1, has been linked to BLCA progression through upregulation by fragile X-related gene 1 (FXR1) [4]. In this study, we explored TRAIP’s role in BLCA, unraveling its nuanced involvement in cellular processes.
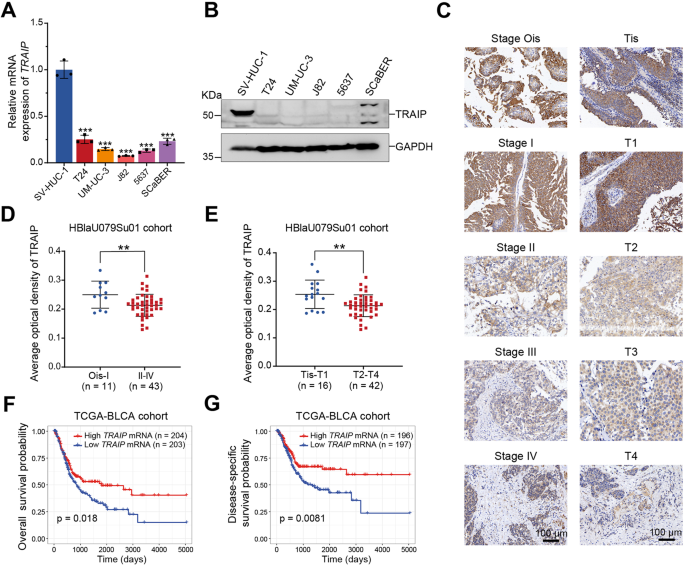
Our investigation commenced by assessing TRAIP expression in BLCA cell lines and normal uroepithelial cells. Intriguingly, we observed higher TRAIP levels in normal cells compared to BLCA counterparts. Subsequent analysis of public databases and our patient samples validated diminished TRAIP expression in BLCA tissues. Immunohistochemical staining on BLCA tissue microarrays further revealed a correlation between reduced TRAIP levels and advanced cancer stages. TCGA database exploration underscored the prognostic significance, indicating poorer outcomes for patients with low TRAIP expression.
Functional experiments involving TRAIP modulation substantiated its pivotal role in inhibiting BLCA proliferation and metastasis. Knockdown of TRAIP intensified these malignancy hallmarks, while TRAIP overexpression exerted suppressive effects. RNA-seq experiments following TRAIP knockdown unraveled its association with the MYC target pathway. Notably, TRAIP emerged as a key regulator of MYC protein stability through ubiquitination, impacting the transcription of MYC target genes.
Employing IP-MS experiments, we identified MYC as a potential direct target of TRAIP. Subsequent Co-IP and GST pull-down assays confirmed their direct interaction, shedding light on TRAIP’s role in regulating MYC protein levels. The overlap of TRAIP and Skp2 binding sites on MYC suggests a potential interplay that warrants further exploration. Mutational analysis pinpointed K428 and K430 as potential TRAIP ubiquitination sites on MYC, aligning with previously reported sites [5].
Exploring the reciprocal relationship, we discovered a significant peak of MYC binding in the TRAIP promoter region, validated by ChIP-qPCR. Luciferase reporter assays reinforced the notion that increased MYC expression stimulates TRAIP transcription. Moreover, manipulating MYC levels in BLCA cells influenced TRAIP mRNA and protein levels, substantiating MYC’s role in TRAIP transcriptional regulation.
In essence, our study elucidates the diminished expression of TRAIP in BLCA progression and its correlation with adverse prognostic outcomes. Unveiling TRAIP’s role in mediating K48-linked polyubiquitination of MYC, leading to its degradation, underscores its tumor-suppressive function. Furthermore, the identified feedback loop, where MYC transcriptionally activates TRAIP, highlights a delicate regulatory mechanism governing optimal MYC levels. Collectively, these findings deepen our understanding of the molecular landscape in BLCA and offer potential avenues for therapeutic exploration.
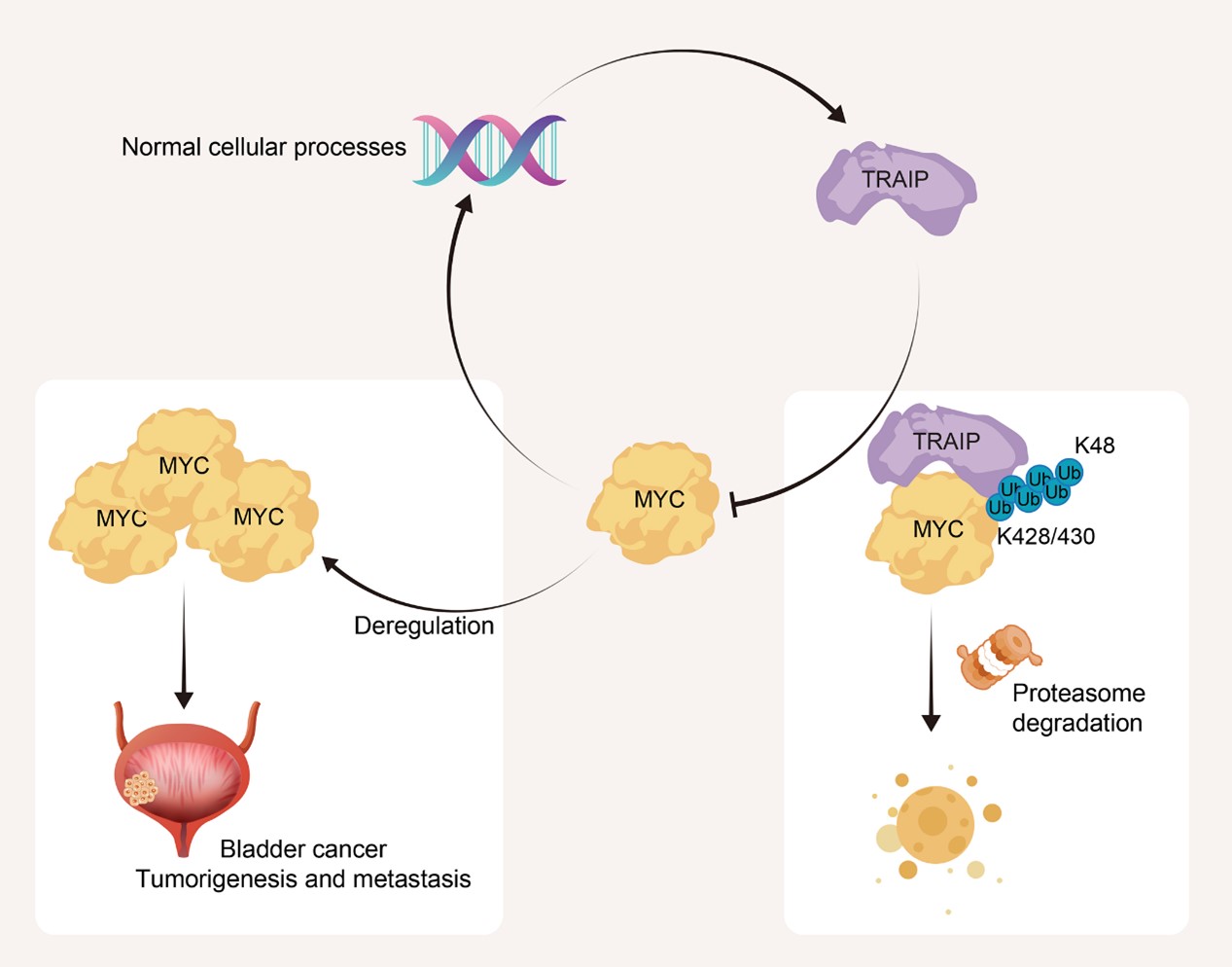
The schematic illustrates negative feedback regulatory mechanism of TRAIP on MYC. MYC can drive the transcription and expression of TRAIP. In turn, TRAIP can interact with MYC and undergo K48-linked polyubiquitination at residues K428/430 of MYC. This leads to the degradation of MYC through the proteasomal pathway. TRAIP regulation of MYC maintains the proper level of MYC in the cell, exerts normal cellular processes, and prevents uncontrolled proliferation and metastasis of BLCA caused by MYC dysregulation.
Dr. Yu Xiao and Dr. Xinghuan Wang are the corresponding authors of this paper, with PhD candidates Jingtian Yu, Mingxing Li and senior researcher Dr. Lingao Ju serving as co-first authors.
References
1 Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71: 209-249.
2 Besse A, Campos AD, Webster WK, Darnay BG. TRAF-interacting protein (TRIP) is a RING-dependent ubiquitin ligase. Biochem Biophys Res Commun 2007; 359: 660-664.
3 Lee SY, Lee SY, Choi Y. TRAF-interacting protein (TRIP): a novel component of the tumor necrosis factor receptor (TNFR)- and CD30-TRAF signaling complexes that inhibits TRAF2-mediated NF-kappaB activation. J Exp Med 1997; 185: 1275-1285.
4 Deng M, Wang N, Li Z, Chen R, Duan J, Peng Y et al. FXR1 can bind with the CFIm25/CFIm68 complex and promote the progression of urothelial carcinoma of the bladder by stabilizing TRAF1 mRNA. Cell Death Dis 2022; 13: 170.
5 Chen Y, Zhou C, Ji W, Mei Z, Hu B, Zhang W et al. ELL targets c-Myc for proteasomal degradation and suppresses tumour growth. Nat Commun 2016; 7: 11057.
Follow the Topic
-
Oncogene

This journal aims to make substantial advances in our knowledge of processes that contribute to cancer by publishing outstanding research.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in