Transforming space life support systems with magnets
Published in Chemistry, Physics, and Mathematical & Computational Engineering Applications
Since the dawn of human spaceflight in the 1960s, efficient oxygen production in space has remained a persistent engineering challenge. While water electrolysis splits water into hydrogen and oxygen, the weightlessness of orbit fundamentally disrupts the process. Gas bubbles stick to electrodes instead of detaching via the buoyant force as they do on Earth, altering mass transport dynamics and requiring complex auxiliary mechanical systems for phase separation.
Current solutions involve heavy, power-intensive equipment prone to mechanical wear and failure. This creates significant obstacles for long-duration missions to Mars and beyond, where system reliability, mass constraints, and power limitations are critical concerns.
Our solution came from a simple idea: using ordinary off-the-shelf permanent magnets to make gas bubbles move in reduced gravitation.
The Physics Behind the Solution
Our breakthrough emerged from recognizing that microgravity conditions (10-6 g) actually disclose certain magnetic properties of aquous solutions that are typically overshadowed by the gravitational force on Earth, namely, the magnetic polarization (MP) force.
MP forces exploit the magnetic susceptibility difference between gas bubbles and the surrounding liquid electrolyte, creating pressure gradients that push bubbles away from electrode surfaces towards the magnet in case of diamagnetic fluids like water.Magnetohydrodynamic (MHD) forces arise from the Lorentz interaction between electric currents and magnetic fields, inducing fluid motion without mechanical stirring. By carefully applying these two forces to electrochemical systems, we were able to build and demonstrate several water-splitting architectures that generate, separate, and collect oxygen and hydrogen bubbles without moving parts or additional power input in microgravity.
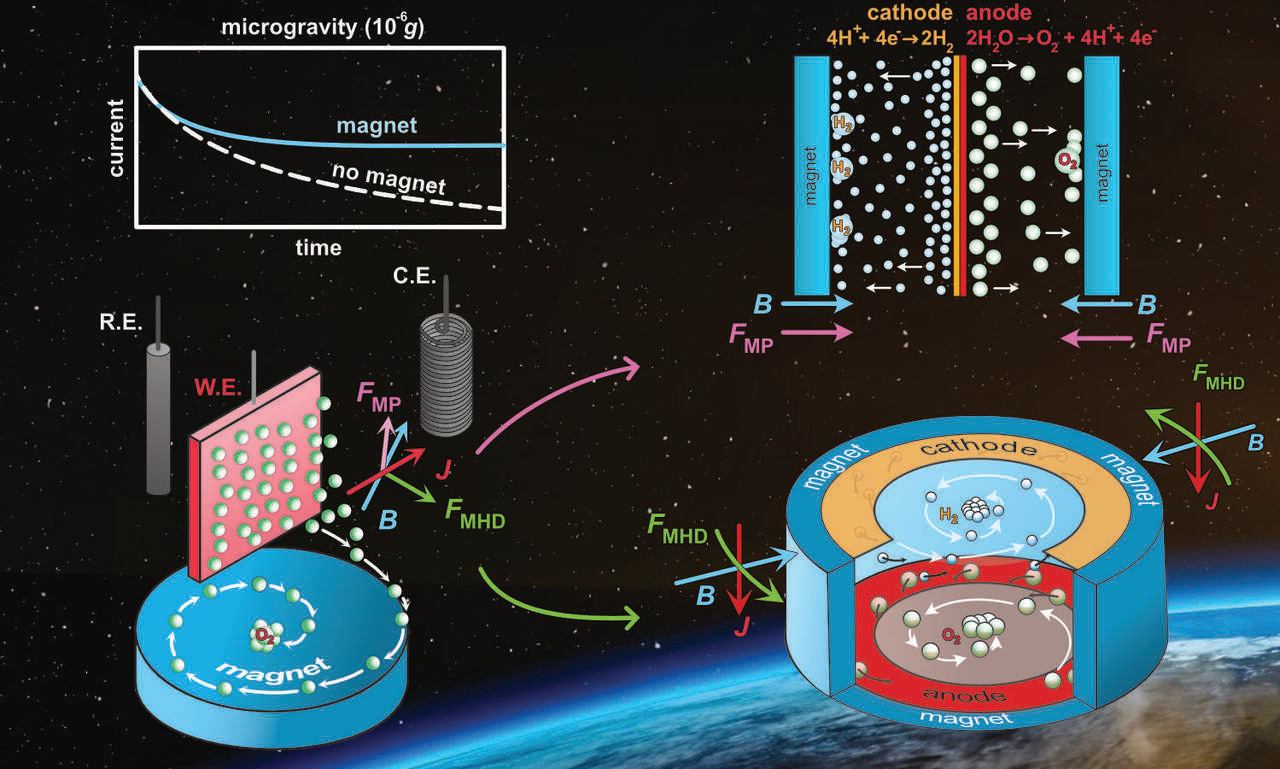
We tested these concepts using mesh-shaped platinum electrodes in the Bremen Drop Tower facility, where microgravity conditions of up to 9.3 s can be generated. The data, shown in Figure 1, also revealed substantial improvements of the current density when magnetic fields were applied, demonstrating that magnetically-driven gas bubble transport can restore near-terrestrial electrolysis efficiency in microgravity.
Three Experimental Approaches
We developed three distinct configurations to validate our theoretical framework, each utlising different magnetic force configurationsfor gas management.
The first approach was a simple electrochemical half-cell setup, carrying out either the hydrogen or oxygen evolution reaction in a three-electrode setup using different electrodes and surface morphologies. Figure 1 represents the data for the hydrogen evolution reaction using a Pt mesh electrode that shows the largest 240% efficiency improvement.
The electrochemical tests of Pt mesh electrodes with and without magnets carried out in microgravity and terrestrial environments is shown in the following video:
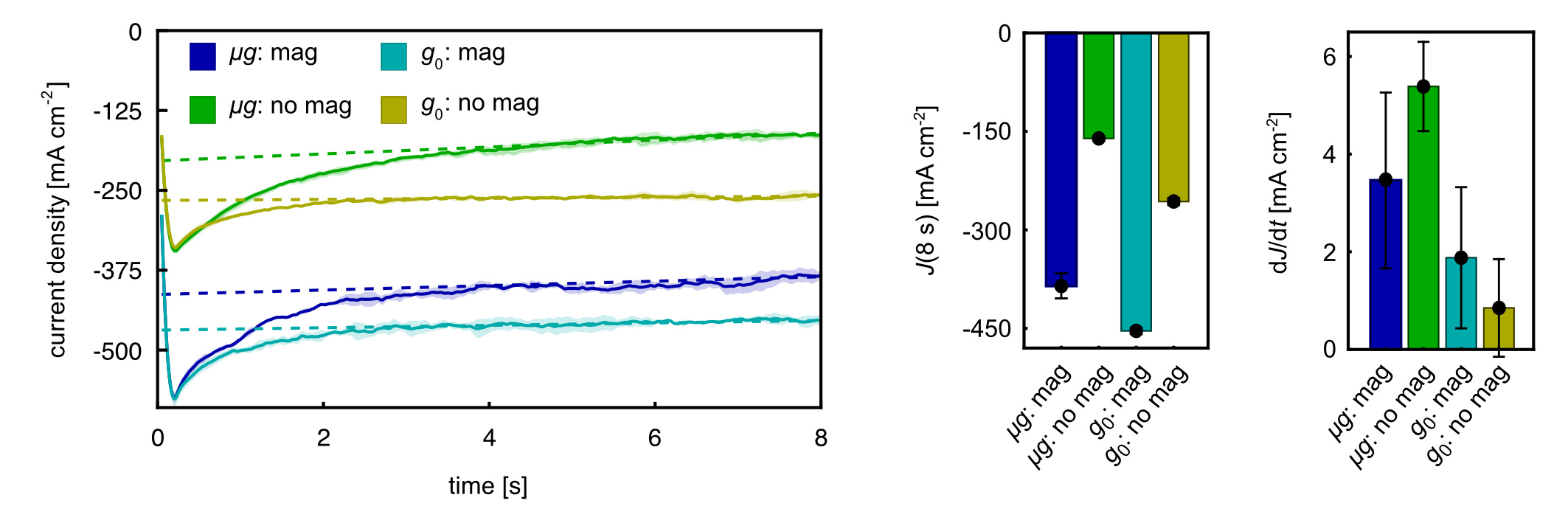
Our proton-exchange membrane (PEM) model electrolyzer, shown in Figure 2, exploits MP forces for passive bubble separation. This configuration uses magnet field gradients to create MP forces that continuously remove bubbles from electrode surfaces. The system operates without additional power input or moving parts, relying entirely on the intrinsic magnetic properties of the liquid.
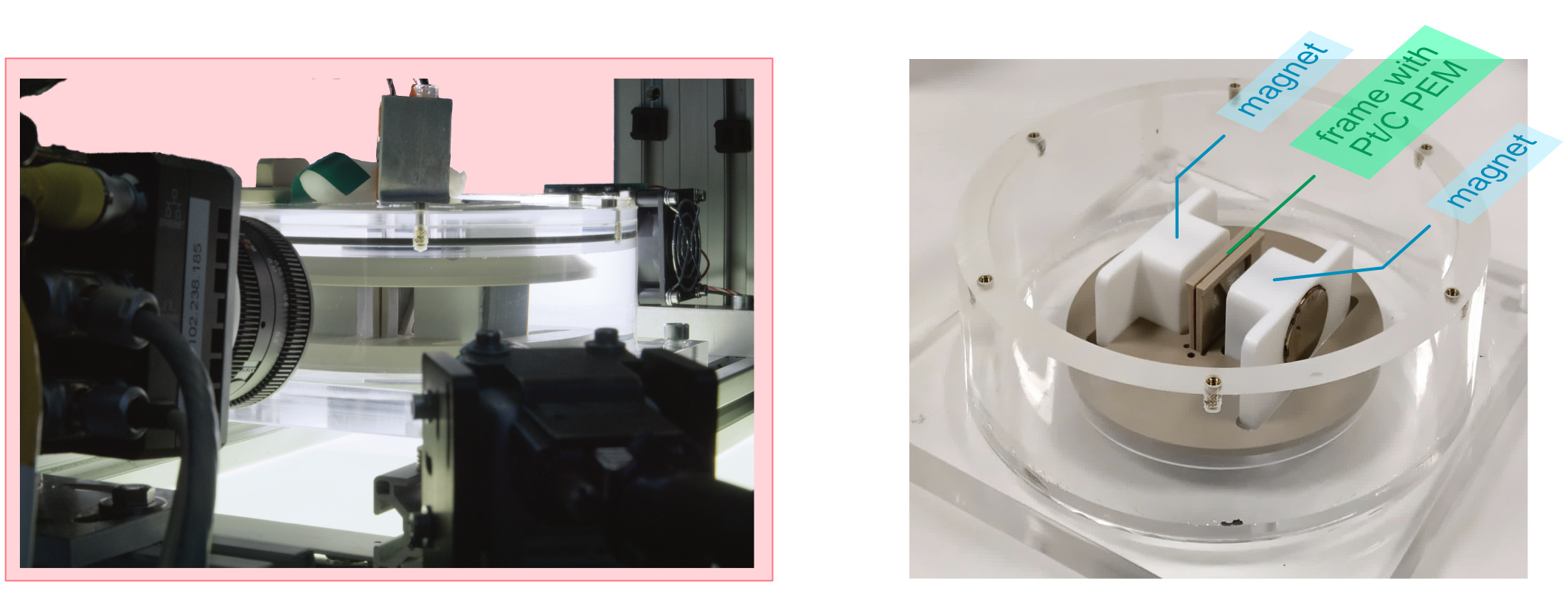
Figure 2: Images of the proton-exchange membrane (PEM) model electrolyser setup equipped with magnets for hydrogen and oxygen gas bubble movement.
A demonstration of our PEM electrolyzer can be seen in the following video:
Another electrolytic design we developed, shown in Figure 3, incorporates a cylindrical MHD drive system that uses the Lorentz force to create a vortex flow. The rotational motion transports gas bubbles towards the central axis through centripetal acceleration, where they can be collected. The system demonstrates how the same electrical current driving electrolysis can simultaneously power the fluid motion necessary for phase separation.
Figure 3: Images of the magnetohydrodynamic (MHD) drive electrolyser setup.
The induced vortex flow and the spiral trajectory of bubbles towards the center is easily observed:
Implications for Space Exploration
This research addresses fundamental limitations in current space life support architectures. Existing systems, while functional, incorporate complex and failure-prone components, possessing high power requirements and extensive maintenance needs that pose significant challenges for long-duration missions.
Our magnetic electrolytic cell architectures offer a promising alternative by eliminating the need for mechanical components and other moving parts for gas-liquid phase separation. The mass and volume savings as well as the reliablity and scalability of the systems could be highly beneficial for future deep space missions.
Future Directions
Our work establishes a new dimensionless parameter that characterize the relative importance of MHD and MP forces in magnetically-actuated phase separation. This framework provides design criteria for optimizing future systems and predicting performance across different operating conditions.
The next phase involves extending our validation to longer microgravity periods through suborbital rocket flights and eventually orbital demonstrations. We are developing full-scale prototypes that could be integrated into future life support systems for orbiting habitats and Mars missions.
Link to Article in Nature Chemistry (https://doi.org/10.1038/s41557-025-01890-0)
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcement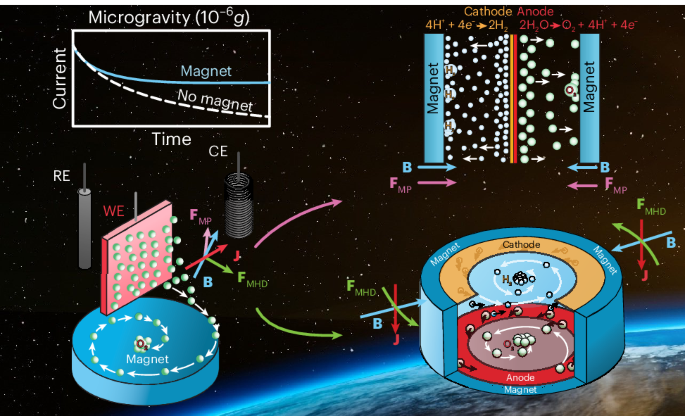




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in