Tuneable mesoporous silica material for hydrogen storage application via nano-confined clathrate hydrate construction
Published in Chemistry and Sustainability
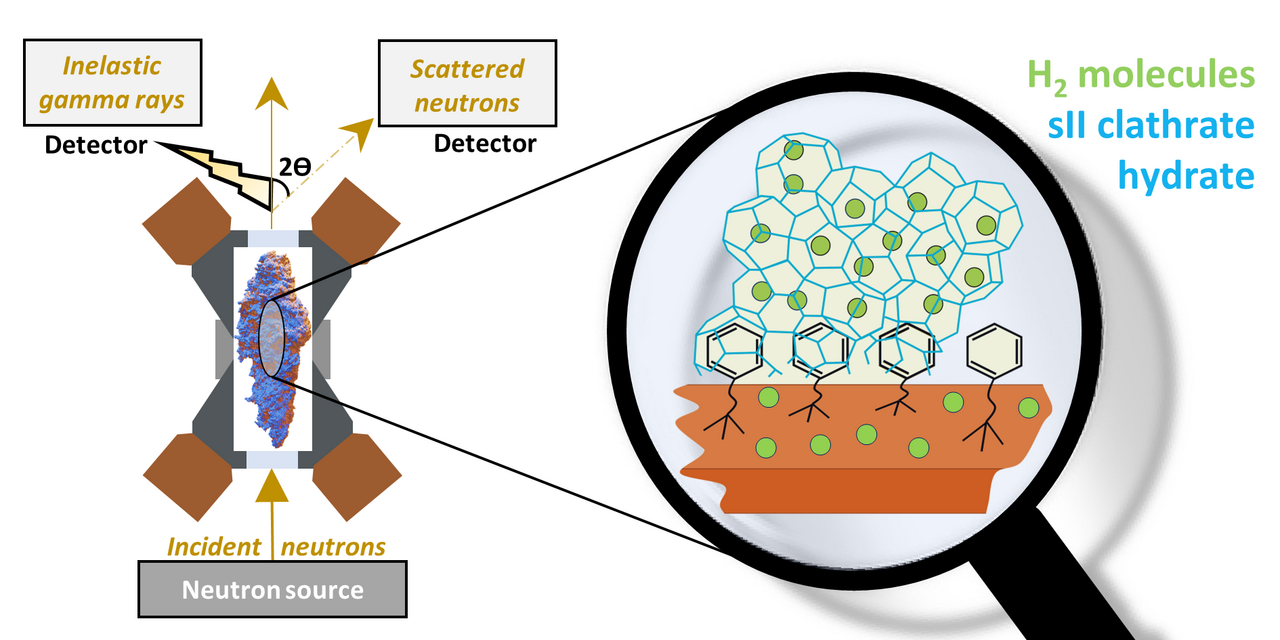
Convenient and safe hydrogen storage is a critical aspect of realizing hydrogen as a viable renewable energy source. Current storage methods (such as compression or liquefaction) are however costly and inefficient. The study aims to explore hydrogen clathrate hydrates, porous ice-like structures capable of trapping and storing hydrogen molecules, storage medium for hydrogen. In bulk, these clathrates require extreme conditions to form, but by confining hydrogen and water together in nanoscale pores of mesoporous silica, the conditions needed to form hydrogen clathrate hydrate are drastically alleviated.
Material and Approach
The research presents a modified mesoporous silica, specifically mesostructured cellular foam (MCF), which is hydrophobically modified to enhance the formation of hydrogen clathrate hydrate, thus increasing storage efficiency. By grafting phenethyl groups onto the silica surface, the hydrophobicity of the material was increased, which in turn has a positive impact on clathrate formation. The hydrophobic surface forces a restructuring of the intermolecular organization of water near the surface, favouring the inclusion of hydrogen molecules and promoting the formation of clathrate hydrate at reduced pressures. Also the selective adsorption of non-polar solutes near the hydrophobized surface renders the otherwise poorly soluble hydrogen molecules more accessible, facilitating clathrate hydrate growth in nano-confined environments.
Experimental Methods
MCF and surface-modified MCFPhene sample were synthesized and characterized using a range of techniques including Fourier-transform infrared spectroscopy (FTIR), nitrogen sorption analysis and high-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) (Figure 1).
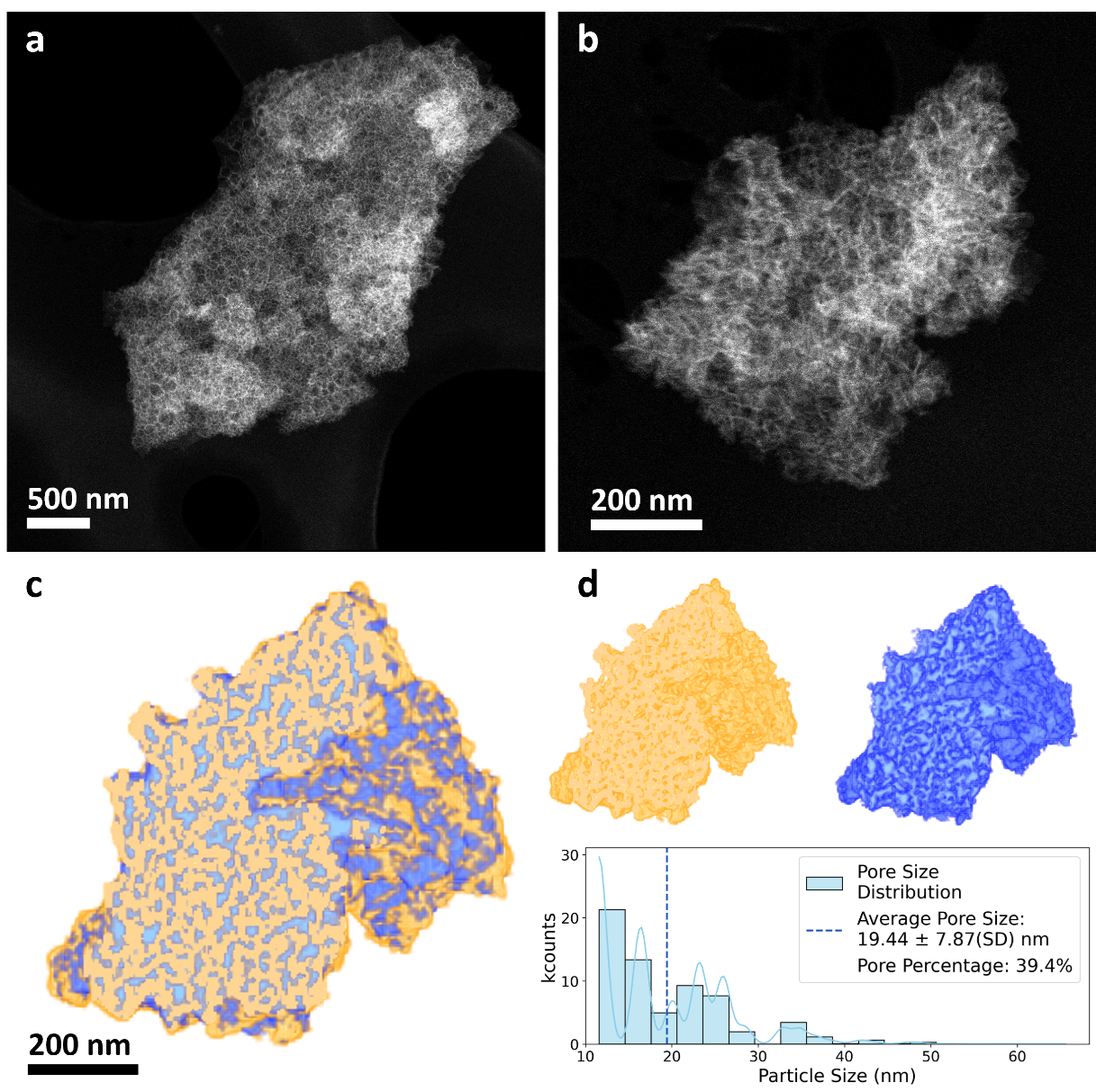
Figure 1. Textural and morphological details of the modified MCF material. (a-b) High-angle annular dark field-scanning transmission electron microscopy (HAADF-STEM) images of two MCFPhene porous particles. (c) Section of the 3D reconstruction volume for the particle in Figure (b), (d) Separate segmentation for the material and pore volumes in yellow and blue colors respectively, together with the segmentation statistics.
The surface-modified silica material was then mixed with D2O and loaded into the sample cell, where it was pressurized and cooled. In-situ inelastic neutron scattering (INS) and neutron diffraction (ND) allowed us to study the interaction between hydrogen and the hybrid silica material, as well as the structure and stability of the formed clathrate hydrates.
Key Results and Findings
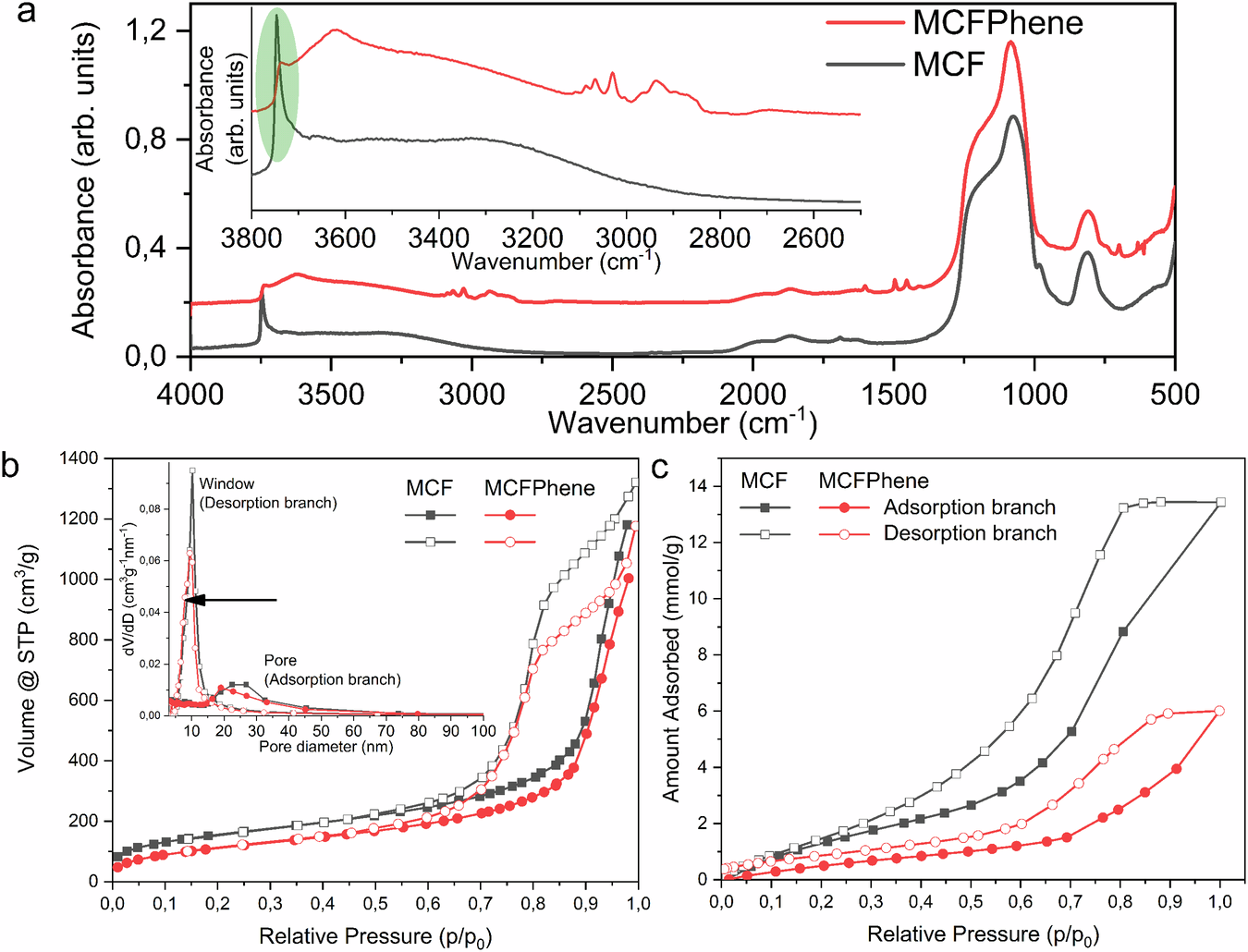
1. Surface Modification: FTIR spectra indicated successful surface modification of the silica material, showing a reduction in hydrophilic silanol groups and the presence of the grafted phenethyl groups. This was crucial in increasing the hydrophobicity of the material.
2. Pore Size and Structure: The nitrogen sorption analysis revealed that the pore size decreased slightly after surface modification, but the mesoporous structure remained intact. Electron microscopy confirmed the presence of uniform large mesopores.
3. Hydrogen Storage Efficiency: The INS experiments showed that hydrogen molecules interact with the surface of the hybrid material at various sites, suggesting the presence of both hydrogen clathrate hydrates and hydrogen adsorbed on the silica walls. The research highlights that two storage sites—clathrate cages and unmodified silica walls—enhance the overall hydrogen storage capacity.
4. Hydrate Formation at Lower Pressures: One of the major achievements of the study is the formation of hydrogen clathrates at 165 MPa (Figure 2 - left), significantly lower than the 200 MPa required for bulk hydrogen clathrate formation. This reduction in pressure by ~20% was attributed to the altered properties of confined water in the nano-pores and the hydrophobic nature of the silica surface.
5. Clathrate Stability: Stability tests showed that the hydrogen clathrates remained stable at temperatures as high as 280 K, well above the solidification temperature of water (Figure 2 - right). This suggests the material’s potential for safe and reliable hydrogen storage, even under practical temperature conditions.
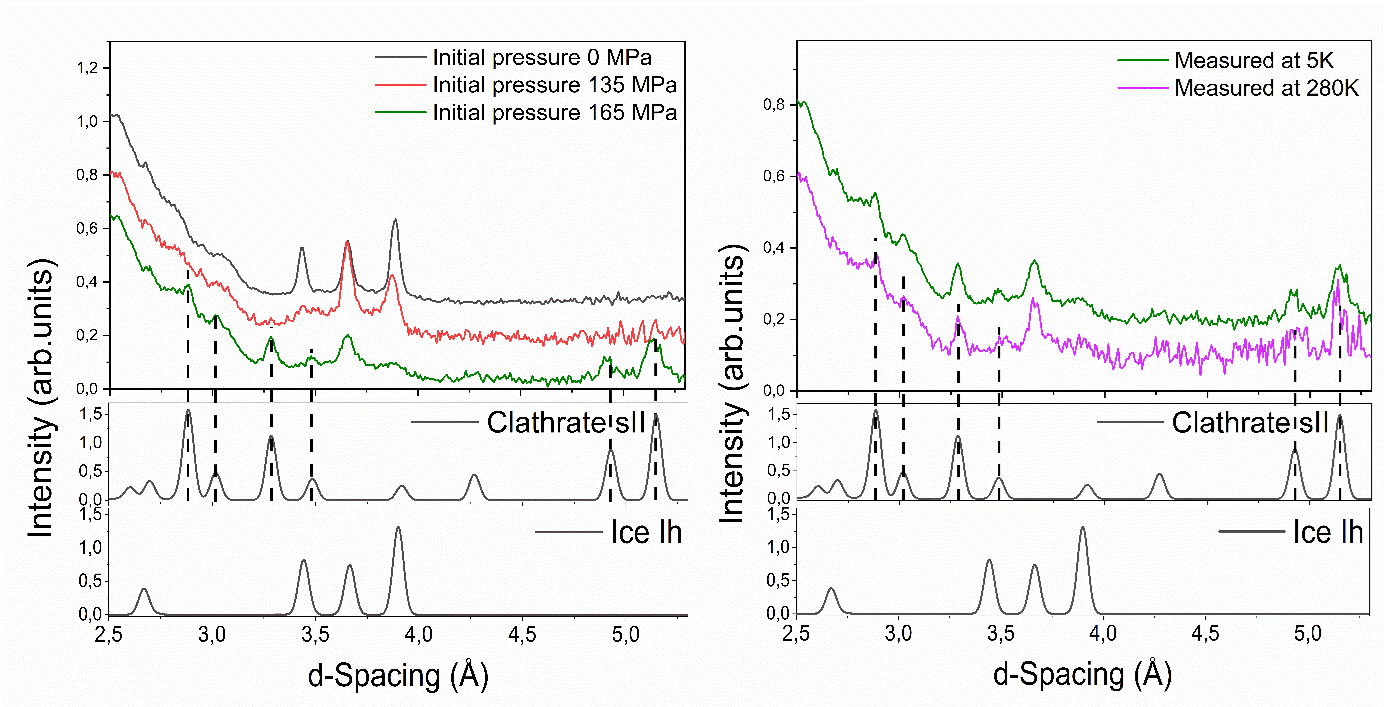
Figure 2. ND data at different initial pressures and temperatures. Neutron diffraction of the MCFPhene-D2O-H2 system after in-situ synthesis at different pressures and measured at 0.1 MPa and 5 K (left). Stability experiments at increased temperature for 165 MPa experiment, measured at 0.1 MPa (right).
Implications and Outlook
This research presents a significant step forward in hydrogen storage technology. By using a surface-modified MCFPhene silica material as porous host, we have successfully reduced the formation pressure for hydrogen clathrates by 20%, demonstrating the potential for more efficient and cost-effective hydrogen storage solutions. Further fine-tuning of surface properties, such as adjusting the hydrophobicity, could improve the efficiency of hydrogen storage even more.
The mechanical stability of these hybrid silica materials under pressures suitable for clathrate formation could open up new pathways towards the reuse of the nano-confined hydrogen (clathrate) hydrate phase in subsequent hydrogen loading-unloading cycles. Multiple-cycles use would also benefit from the so-called “memory effect” typically observed in clathrate science, where the material exhibits enhanced clathrate formation after the first storage cycle, suggesting potential improvements in hydrogen loading and unloading kinetics over multiple uses. Future work will focus on exploring other surface modifiers and optimizing the conditions for large-scale hydrogen storage applications.
In summary, the manuscript provides a comprehensive exploration of how surface modification and nanoconfinement in mesoporous silica can facilitate the formation of hydrogen clathrates at significantly lower pressures and higher temperatures, thus offering a novel approach to tackling the challenges of hydrogen storage.
Acknowledgements
This work is a result of the ARCLATH-1 and ARCLATH-2 moonshot projects (https://www.moonshotflanders.be), consortium projects involving the UAntwerpen, KU Leuven, UGent and VUB. These projects were funded by the Flemish agency for innovation and entrepreneurship (VLAIO), to stimulate development of breakthrough technologies enabling faster decarbonisation of the Flemish economy.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in