Tuning the Zeolite Acidity Enables Selectivity Control by Suppressing Ketene Formation in Lignin Catalytic Pyrolysis
Published in Chemistry
Converting lignin into valuable chemicals and fuels has attracted extensive attention by chemists due to its aromatic-rich structure and large natural abundance. The complex molecular structure of lignin, however, leads to a broad product distribution, which means high costs for downstream processes. Understanding the reaction mechanism during catalytic lignin depolymerization will open up new avenues to rational catalyst design and process optimization to control the selectivity of this process.
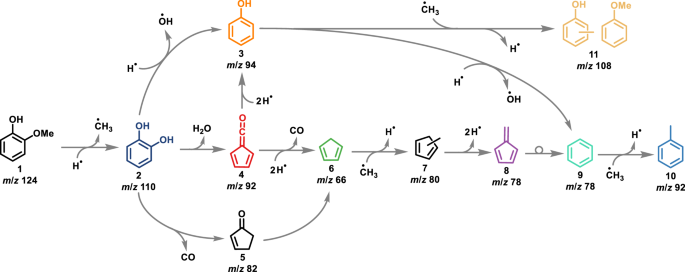
Our groups investigated many lignin model compounds, ranging from benzenediols to dimers, in (catalytic) pyrolysis using operando photoelectron photoion coincidence (PEPICO) spectroscopy to obtain deep mechanistic insights in a bottom-up strategy. [1-6] PEPICO has been established as a versatile operando tool to detect and quantify reactive intermediates in heterogenous catalysis by a unique combination of mass spectrometry and photoelectron spectroscopy.[7, 8] According to the literature and our group’s work, a comprehensive understanding of catalytic pyrolysis of e.g. guaiacol (Fig. 1), one of three main basic units in the lignin structure, was achieved.[1-3, 9, 10]
The mechanism of guaiacol 1 is initiated by a demethylation yielding catechol 2 and methyl and branches between a direct dehydroxylation reaction to phenol 3, and formation of fulvenone 4, a highly reactive ketene intermediate, via dehydration. Formation of ketene 4 is accelerating the reaction rates, which also produces phenol 3 but also cyclopentadiene 6, fulvene 8 and other species, lowering the selectivity of this process.
Since the dehydroxylation 2 -> 3 is a Brønsted acid catalyzed reaction, increasing the concentration of these sites will likely boost the phenol 3 selectivity by making it more likely that catechol 2 will coordinate on two acid sites simultaneously, thereby isolating the hydroxyl groups and suppressing fulvenone 4 formation via dehydration. This should increase the selectivity towards dehydroxylation to phenol 3.
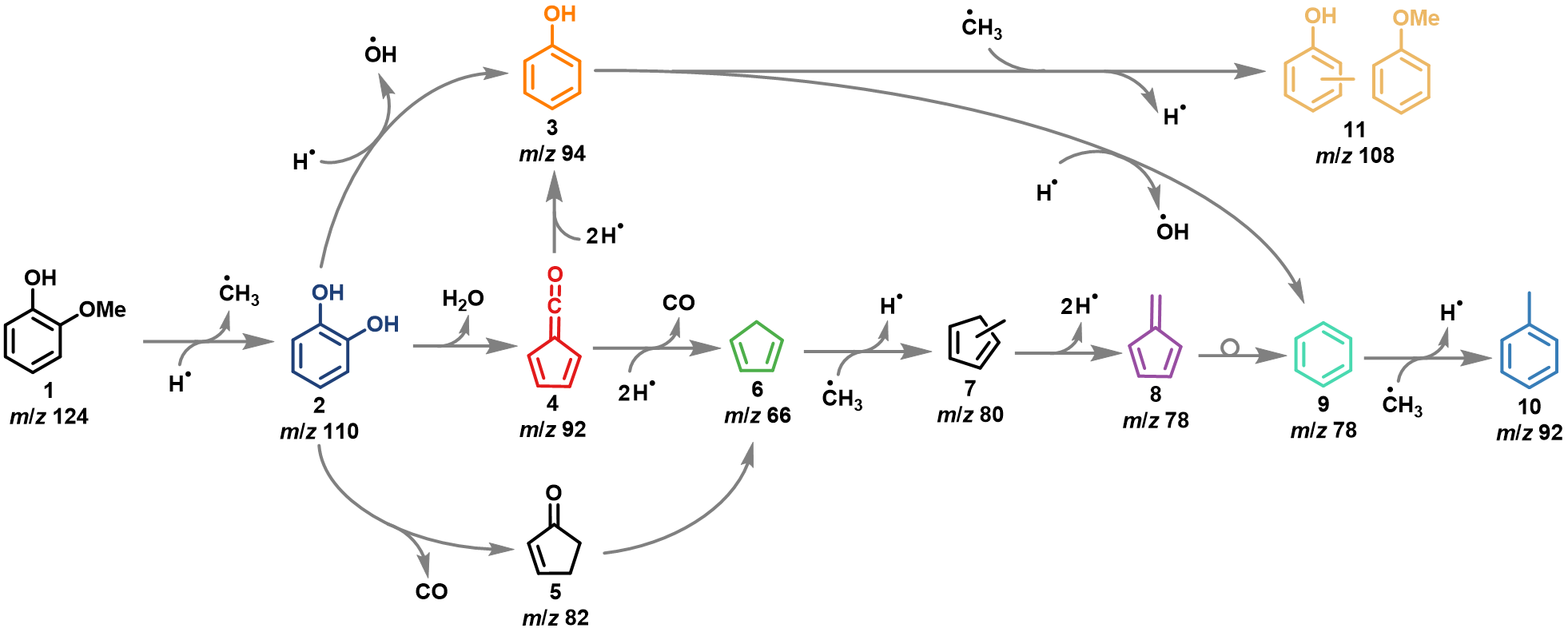
Fig. 1 | Reaction mechanism of guaiacol catalytic fast pyrolysis using HZSM-5
We tested this hypothesis by lowering the Si/Al ratio in the zeolite, which increases the Brønsted acid site density and utilized operando PEPICO spectroscopy as analytical tool, enabling ketene detection.
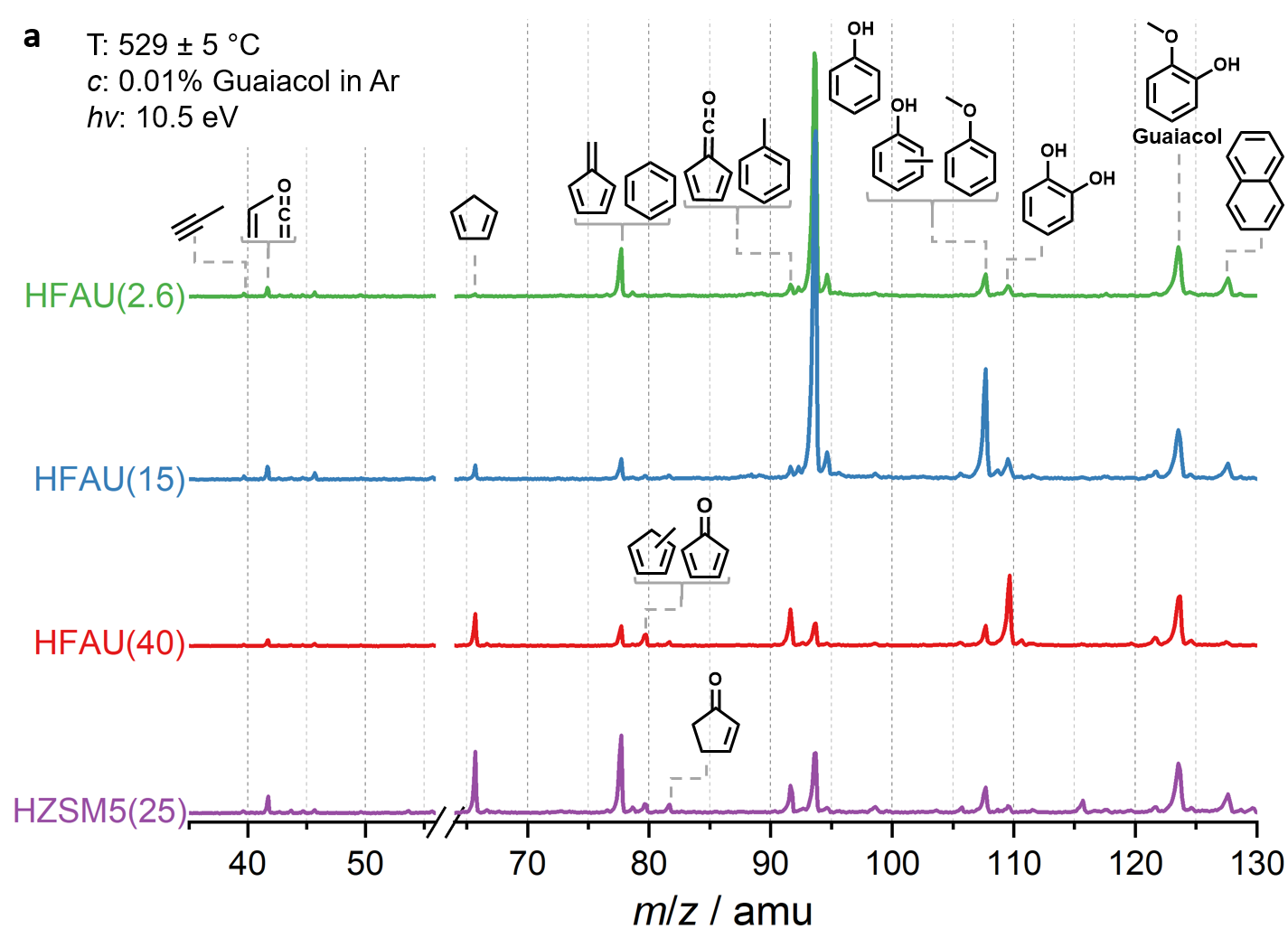
Fig. 2 | Mass spectra of the catalytic fast pyrolysis products of guaiacol over zeolite catalysts.
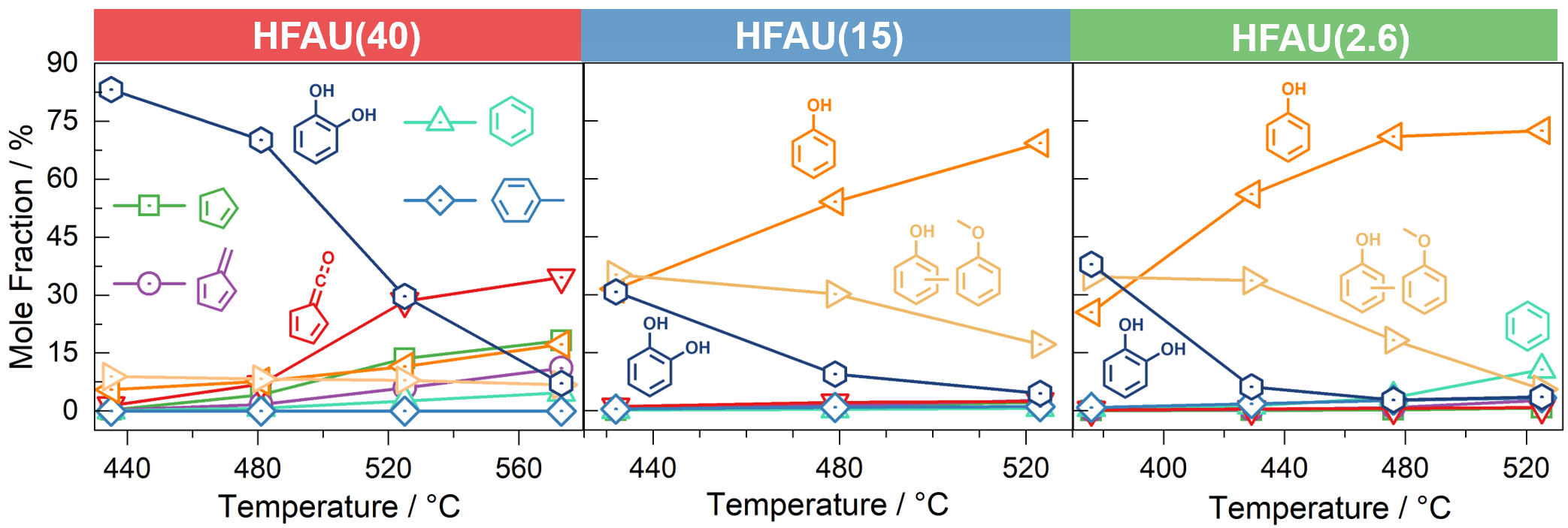
Fig. 3 | Temperature dependent mole fraction of the main products and intermediates in guaiacol CFP.
Mass spectra in Fig. 2 at the same reaction conditions show that the phenol selectivity is strongly increased in HFAU(2.6) and HFAU(15), while the product distribution of HFAU(40) is less selective. By quantification of intermediates and products (Fig. 3), we found that HFAU(15 and 2.6) favors the phenol production, while fulvenone is strongly suppressed. To understand how HFAU catalysts with different Si/Al ratios affect the reaction mechanism, we traced the central intermediates fulvenone and fulvene via photoion mass-selected threshold photoelectron spectroscopy (ms-TPES), as shown in Fig. 4. Fulvenone and fulvene dominate in the HFAU(40) experiment, while they are almost fully suppressed in HFAU(2.6). This result proves that HFAU(15) and HFAU(2.6) suppress the fulvenone production upon catalytic pyrolysis of guaiacol and is the key to increase the selectivity of the reaction.
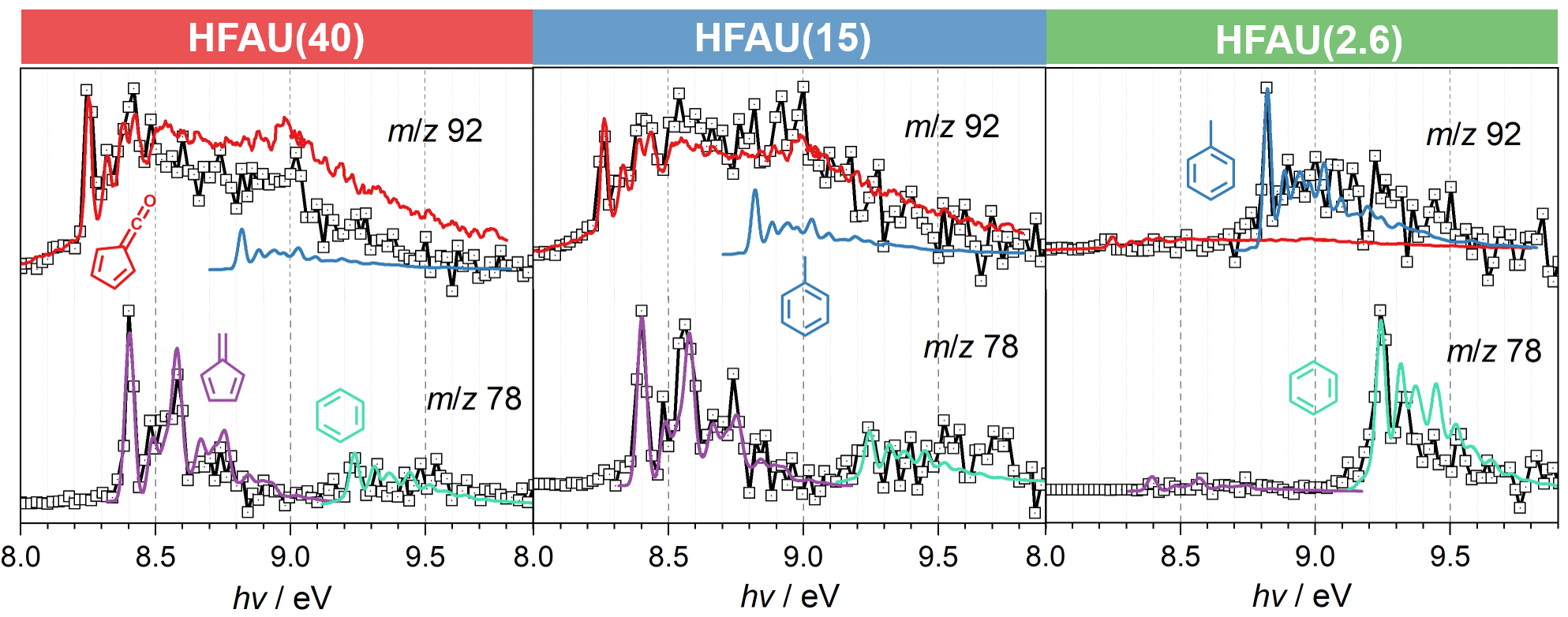
Fig. 4 | Comparison of the experimental m/z 78 and 92 ms-TPES (squares) with reference spectra (colored lines) of spectral carriers.
This can be understood as outlined in Fig. 5. Brønsted acid sites are the main active sites in guaiacol catalytic pyrolysis. As HFAU(40) exhibits a high Si/Al ratio and thus low density of Brønsted acid sites, the initial product catechol is only coordinated to a single Brønsted acid sites (Fig. 5 top). The two hydroxyl groups can interact with each other favoring the intramolecular dehydration of catechol and fulvenone formation. Due to the high reactivity of this elusive intermediate the reaction is hardly controllable, leading to an unselective formation of many different products. In contrast, the two hydroxyl groups in catechol are isolated on HFAU(15) and HFAU(2.6) due to the bidentate bonding within the zeolite pore, which suppresses the fulvenone production, leading to a dominant selectivity towards phenol. In addition to detection of reactive intermediates and quantification of the products, we performed reaction pathway calculations as well as 29Si MAS-NMR spectroscopy to verify the reaction mechanism.
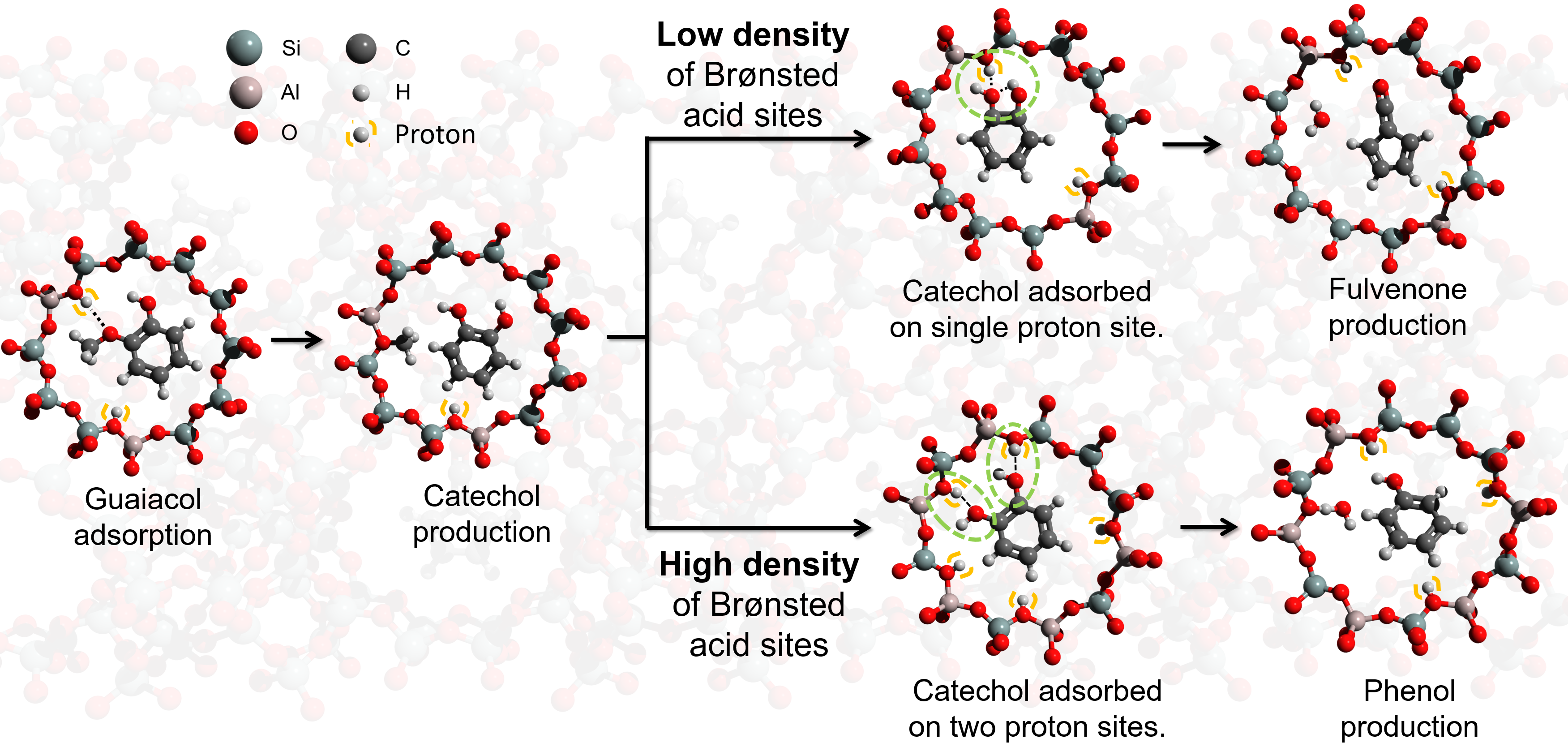
Fig. 5 |The effect of Brønsted acid site density on the reaction mechanism of guaiacol catalytic pyrolysis
In conclusion, by linking reactive intermediate concentrations, selectivities and the conversion, it has been shown that the guaiacol catalytic pyrolysis can be optimized in a targeted way. This approach is broadly applicable to many heterogeneous catalytic processes, ranging from hydrogenation and syngas- or methanol-to-hydrocarbon reactions. Taking control of ketenes and their surface analogs may have additional benefits for the overall selectivity, especially for MTH reactions. Our operando PEPICO approach can assist rational catalyst design to control product selectivities for targeted process optimization.
[1] Hemberger, P., et al. Understanding the mechanism of catalytic fast pyrolysis by unveiling reactive intermediates in heterogeneous catalysis. Nature communications, 2017, 8(1), 15946.
[2] Pan, Z., et al. Isomer-dependent catalytic pyrolysis mechanism of the lignin model compounds catechol, resorcinol and hydroquinone. Chemical science, 2021, 12(9), 3161-3169.
[3] Pan, Z., et al. Operando PEPICO unveils the catalytic fast pyrolysis mechanism of the three methoxyphenol isomers. Physical Chemistry Chemical Physics, 2022, 24(36), 21786-21793.
[4] Wu, X., et al. Isomer-Dependent Selectivities in the Pyrolysis of Anisaldehyde. Energy & Fuels, 2022, 36(13), 7200-7205.
[5] Gerlach, M., et al. Metamorphic meta isomer: carbon dioxide and ketenes are formed via retro-Diels–Alder reactions in the decomposition of meta-benzenediol. Physical Chemistry Chemical Physics, 2019, 21(35), 19480-19487.
[6] Wu, X., et al. Unimolecular thermal decarbonylation of vanillin stifled by the bimolecular reactivity of methyl-loss intermediate. Journal of Analytical and Applied Pyrolysis, 2022, 161, 105410.
[7] Hemberger, P., et al. Photoelectron photoion coincidence spectroscopy provides mechanistic insights in fuel synthesis and conversion. Energy & Fuels, 2021, 35(20), 16265-16302.
[8] Hemberger, P., et al. New analytical tools for advanced mechanistic studies in catalysis: photoionization and photoelectron photoion coincidence spectroscopy. Catalysis Science & Technology, 2020, 10(7), 1975-1990.
[9] Liu, P., et al. Exploring the reaction chemistry of biomass upgrading over HZSM-5 catalyst through model compounds. Fuel, 2022, 312, 122874.
[10] Jiang, X., et al. Catalytic conversion of guaiacol as a model compound for aromatic hydrocarbon production. Biomass and Bioenergy. 2018, 111, 343–351.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in