Two-way workable solution to infarcted heart: Hydrogel suture as a patch and sensor for monitoring, diagnosing and repairing
Published in Bioengineering & Biotechnology

Our lives hinge on organs and tissues to work accurately around the clock. They can fail when damaged, and organ transplantation is needed. However, donor organ scarcity leads to the increasing demand for repairing and regeneration. Heart disease is a leading cause of death and contributes a substantial burden to our society. Heart attack is unexpected but comes about instantly. During MI, microcirculation disturbance in the ischemic area leads to tissue necrosis and fibrous tissue forming, leading to malignant arrhythmia and myocardial remodeling, affecting the function of the myocardium and reducing the quality of life of patients. How, where, and when are three critical elements in treating and understanding complex organ injuries. The "diagnosis-treatment-monitoring" procedure for damaged organs still lacks a convenient and effective scheme.
Recently, functional suture had enormous potential for real-time diagnosis, evaluation, and treatment of damaged organs and tissues along its wound closure task. These sutures can realize wireless sensing, drug elution, near-infrared light heat conversion, inhibition of bacterial breeding and sensing functions. However, although these sutures can provide flexible treatment schemes, their single function in monitoring the disease’s progress of deep tissues is still limited, and it is impossible to integrate multiple monitoring schemes and provide flexible treatment.
Here, we report an intelligent hydrogel suture for heart repair (Fig. 1a-d). The elasticity, low friction, and stiffness of the hydrogel sutures ensure that it can maintain good mechanical properties to adapt to the tension of the wound edema, and after the edema subsides, the original tension can be restored. Moderate elasticity avoids the potential risk of tissue ischemia and necrosis caused by sutures. At the same time, the micro-flow channel of the hydrogel suture also provides a two-way drug delivery and body fluid signal acquisition pathway. By easily adjusting the composition of the hydrogel sutures, the hydrogel is endowed with additional functions, such as photothermal effects and force luminescence. Thus, while the DTMS with microfluidic tract has capillary phenomena, the photothermal effect of DTMS provides a method to kill pathogens extensively based on physical methods, avoiding the potential risk of infection caused by DTMS allowing fluid flow through the tissue barrier. And Force-induced luminescence hydrogel sutures can convert stress stimuli into an optical signal and can be used as potential novel sensors to detect life signals such as motion, respiration, and pulse.
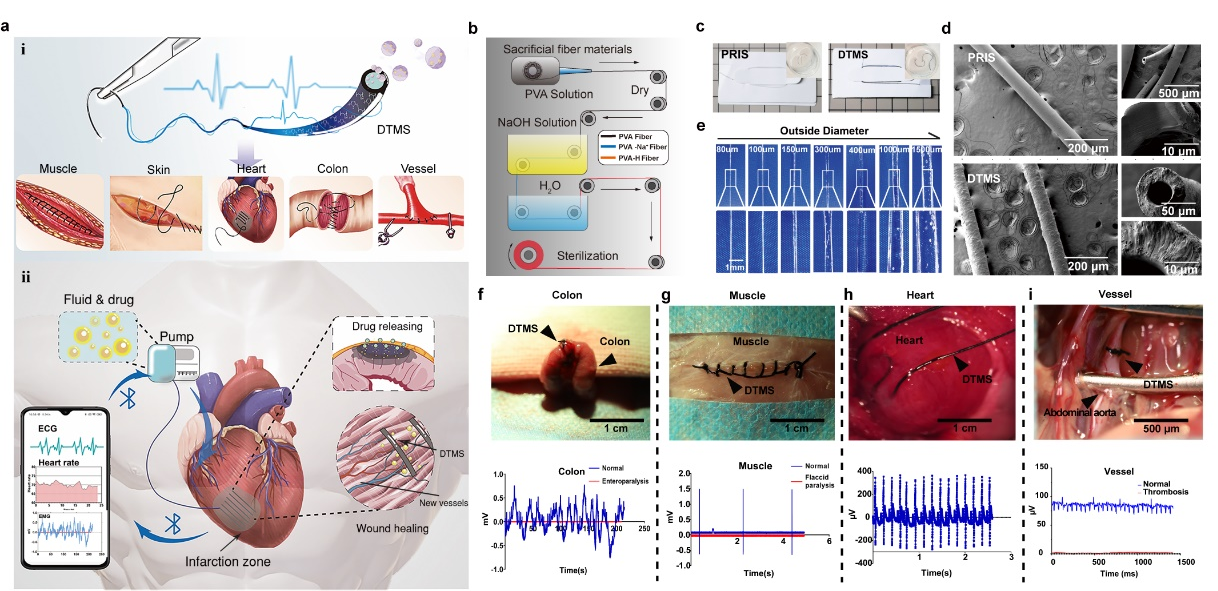
Figure 1: Concept design, preparation, and physical characterization of DTMS
a The diagnosis, treatment, and monitoring suture (DTMS) in scheme for different tissues (i), DTMS conducting signals of infarcted hear and deliver drugs on demand (ⅱ). b Manufacturing process of PVA hydrogel suture. c DTMS and PRIS (Primary Suture). d Scanning electron microscope (SEM) of DTMS and PRIS (Primary Suture), n=4 independently replications with similar results. e Transparent PRIS with outer diameter (OD) of 80 μm, 150 μm, 300 μm, 400 μm, 1000 μm, 1.5 mm and inner diameter (ID) of 50 μm, 80 μm, 150 μm, 300 μm, 400 μm, 1000 μm. f DTMS and Bluetooth module sensing the electrophysiological waveform of intestinal peristalsis and detecting paralysis. g DTMS and Bluetooth module sensing in the skeletal muscle sensing the electromyography (EMG) of normal muscle and detecting soft paralysis. h DTMS and Bluetooth module in the myocardium for electrocardiogram (ECG) signal sensing. i DTMS in the rat abdominal aorta to monitor electrophysiological signals of vascular pulsation and detect vascular occlusion.
Conductive hydrogels carrying microfluidic channels have tunable dimensions and biosafety, drug-controlled release, and sensors in the deep diseased tissue. In rats, DTMS sutures can detect the muscle action potential and identify intestinal paralysis, thrombosis, and myocardial infarction (Fig1. f-i). The difference between DTMS and the components of biosensors such as patches is that DTMS can be precisely combined with the tissue, reducing the noise caused by large-area electrode patches, and avoiding signal interference caused by poor binding, and is more sensitive and accurately reflects the electrophysiological characteristics of the tissue. Good electrical conductivity and a wide range of application scenarios provide evidence that DTMS can be used as a hydrogel electrode with good biological affinity, with great monitoring and diagnostic potential for deep complex soft tissues and organs.
As far as we know, this work is the first known application of drug-delivery sutures in the treatment of myocardial infarction. In both rat and porcine MI models, DTMS restored cardiac function by providing a favorable mechanical environment for the myocardium and delivered to accelerate the repair process. All of these results suggest that DTMS and the nitric oxide prodrugs can inhibit the inflammatory response and recruit repair-related macrophages in the early stages of trauma, reducing oxidative stress caused by inflammation and ischemia, and thereby promoting tissue recovery. In the pig myocardial infarction model, DTMS effectively reduced the area of transmural myocardial infarction and improved abnormal ventricular remodeling to save cardiac function. This indicates that DTMS plays a role in diagnosing, treating, and reducing the incidence of malignant cardiovascular complications in the treatment of cardiac ischemic diseases.
In conclusion, the DTMS can be compact and flexibly used to judge the occurrence and prognosis of the disease, and to deliver the drugs into the lesion timely and accurately. It has important significance for the diagnosis of the condition outcome after treatment.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in